Summary
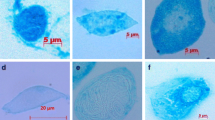
This study provides information relevant to future research aimed at producing Limulus Amebocyte Lysate (LAL) in vitro, which would potentially reduce the need to harvest and bleed horseshoe crabs as in the current methods of LAL production. To address the need for primary culture of horseshoe crab amebocytes, this study tested the effects of a variety of standard insect cell culture media on amebocyte morphology and viability after 7 d of maintenance. Amerbocyte morphology was least altered from in vivo form in Grace’s Modified Insect Medium, with no observed degranulation of cells, as compared to the other media tested. There were significant differences in amebocyte viability among the six insect cell culture media tested. Grace’s Modified Insect Medium sustained viability of 77.2±5.1% (mean ± standard deviation) of amebocytes, followed distantly by Grace’s Insect Medium with 35.1±8.7% amebocyte viability. Results indicate that Grace’s Modified Insect Medium with horseshoe crab serum supplementation was the best candidate of the six media tested for future medium optimization for Limulus amebocyte requirements.
Similar content being viewed by others
References
Armstrong, P. B. Adhesion and motility of the blood cells of Limulus. In: Cohen, E. ed. Blood cells of marine invertebrates: experimental systems in cell biology and comparative physiology. New York: Alan R. Liss 1985; 77–124.
ASMFC (Atlantic States Marine Fisheries Commission). Fishery management report no. 32b—addendum II to the interstate fishery management plan for horseshoe crab. Washington, D.C.: Atlantic States Marine Fisheries Commission; 2001.
Chen, I.-J.; Chen, Y.-M.; Hong, S.-J.; Chiang, L.-C. Morphological changes of horseshoe crab amebocytes during in vitro cultivation. Kaohsiung J. Med. Sci. 2:769–773; 1986.
Chen, I.-J.; Hong, S.-J.; Chen, Y.-M.; Yang, Y.-C. Cultivation of horseshoe crab amebocytes. Kaohsiung J. Med. Sci. 5:515–521; 1989.
Copeland, E.; Levin J. The fine structure of the amebocyte in the blood of Limulus polyphemus. I. Morphology of the normal cell. Biol. Bull. 169:449–457; 1985.
Ding, J. L.; Ho, B. A new era in pyrogen testing. Trends Biotechnol. 19(8):277–281; 2001.
Ding, J. L.; Kim, J. C.; Ho, B. Pokeweed mitogen stimulates DNA synthesis in cultured amoebocytes of Carcinoscorpius rotundicauda. Cytobios 55:147–154; 1988.
Freshney, I. R. Culture of animal cells: a manual of basic technique. New York: Alan R. Liss; 1987.
Frieberg, J. A.; Weathers, P. J.; Gibson, D. G., III Culture of amebocytes in a nutrient mist bioreactor. In Vitro Cell. Dev. Biol. 28A(3):215–217; 1991.
Gibson, D. G., III; Hilly, J. B. Patent no. 5,082,782: production of horseshoe crab amebocytes in vitro. Washington, DC: U.S. Patent and Trademark Office; 1992.
HCTC (Horseshoe Crab Technical Committe). Status of the horseshoe crab (Limulus polyphemus) population of the Atlantic coast. Washington, D.C.: HCTC, Atlantic States Marine Fisheries Commission; 1998.
Hurton, L. Reducing post-bleeding mortality of horseshoe crabs (Limulus polyphemus) used in the biomedical industry. M.S. thesis. Virginia Polytechnic Institute and State University; Blacksburg, VA: 2003.
Joshi, B.; Chatterji, A.; Bhonde, R. Long-term in vitro generation of amoebocytes from the Indian horseshoe crab Tachypleus gigas (Müller). In Vitro Cell. Dev. Biol. 38A:255–257; 2002.
Kurz, W.; James-Pirri, M. J. The impact of biomedical bleeding on horseshoe crab, Limulus polyphemus, movement patterns on Cope Cod, Massachusetts. Mar. Fresh. Behav. Physiol. 35:261–268; 2002.
Levin, J.; Bang, F. B. The role of endotoxin in the extracellular coagulation of Limulus blood. Bull. Johns Hopkins Hosp. 115:265; 1964.
Mather, J. P., Roberts, P. E. Introduction to cell and tissue culture. New York: Plenum Press Publishing; 1998.
Mikkelsen, T.: The secret in the blue blood. Beijing: Science Press; 1988.
Mitsuhashi, J. Invertebrate cell culture methods. New York: Springer; 2002.
Novitsky, T. J. Diseovery to commericalization: the blood of the horseshoe crab. Oceanus 27(1):13–18; 1991.
Pearson, F. C. Patent no. 4,229,541; In vitro cultivation of horseshoe crab amebocytes. Washington, DC: U.S. Patent and Trademark Office; 1980.
Pearson, F. C.; Woodland, E. The in vitro cultivation of Limulus amebocytes. In: Cohen, E., ed. Biomedical applications of the horseshoe crab (Limulidae). New York: Alan R. Liss; 1979: 93–102.
Rudloe, A. The effect of heavy bleeding on mortality of the horseshoe crab, Limulus polyphemus, in the natural environment. J. Invertebr. Pathol 42:167–176; 1983.
Schrading, E.; O’Connell, T. J.; Michels, S.; Perra, P. Interstate management plan for horseshoe crab. Washington, DC: Atlantic States Marine Fisheries Commission; 1998.
Sherman, R. G. Chelicerates. In: Ratcliffe, N. A., Rowley, A. F., ed. Invertebrate blood cells. New York: Academic Press, 1981:355–384.
Smith, S. A.; Berkson, J.; Barratt, R. A. Horseshoe crab (Limulus polyphemus) hemolymph biochemical and immunological parameters. Proceedings of the 33rd Annual Conference of the International Association for Aquatic Animal Medicine. Albufeira, Portugal, May 4–8, 2002.
Suhr-Jessen, P.; Baek, L.; Jakobsen, P. P. Microscopical, biochemical, and immunological studies of the immune defense system of the horseshoe crab, Limulus polyphemus. Biol. Bull. 176:290–300; 1989.
Thompson, M. Assessments of the population biology and critical habitat for the horseshoe crab, Limulus polyphemus, in the South Atlantic Bight. M.S. thesis. University of Charleston, South Carolina; 1998.
Walls, B. A.; Berkson, J. M. Blood extraction effects on horseshoe crabs (Limulus polyphemus). Fishery Bull. 101:457–459; 2003.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hurton, L.V., Berkson, J.M. & Smith, S.A. Selection of a standard culture medium for primary culture of Limulus polyphemus Amebocytes. In Vitro Cell.Dev.Biol.-Animal 41, 325–329 (2005). https://doi.org/10.1007/s11626-005-0003-5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11626-005-0003-5