Abstract
Background
Treatment-seeking people with opioid use disorder (OUD) who are capable of pregnancy need accurate information about the potential impact of medication to treat OUD (MOUD) on fertility to make informed choices about treatment that are consistent with their reproductive wishes. There is a dearth of research on fertility associated with MOUD receipt in birthing people with OUD.
Objective
To estimate the association between treatment with MOUD and odds of conception among birthing people using national administrative claims.
Design
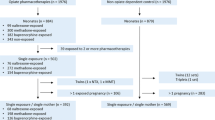
Retrospective case-crossover study using multi-state US administrative data (2006–2016). Dates of conception were estimated from delivery dates and served as “case” days for which MOUD exposures were compared to those on all other (“control”) days of insurance enrollment.
Participants
Treatment-seeking people with OUD with a delivery during the observation period.
Main measures
Odds ratios for conception from within-person fixed effects models were modeled as a function of exposure to MOUD (buprenorphine, methadone, extended-release depot naltrexone, or oral naltrexone) using conditional logistic regression.
Key Results
A total of 21,928 births among 19,133 people with OUD were identified. In the sample, 5873 people received buprenorphine, 1825 methadone, 486 extended-release naltrexone, and 714 oral naltrexone. Participants could receive more than one type of MOUD. Mean age was 28.2 years (SD = 2.2; range = 16–45), with 76.2% having Medicaid. vs. commercial insurance. Compared to no MOUD, periods of methadone (aOR = 0.55 [95% CI = 0.48–0.63]) or buprenorphine receipt (aOR = 0.84 [0.77–0.91]) were associated with fewer conceptions. Treatment periods with extended-release depot naltrexone compared to no medication were associated with higher odds of conception (aOR = 1.75 [1.22–2.50]) and there was no significant difference in conception with oral naltrexone (aOR = 1.02 [0.67–1.54]).
Conclusions
The association between MOUD and odds of conception among birthing people varied by type of MOUD, with extended-release naltrexone associated with higher odds of conceiving compared to no treatment. Clinical studies are urgently needed to investigate these findings further.

Similar content being viewed by others
Data Availability
No additional data available. We intend to provide relevant code on written reasonable request.
References
Heil SH, Jones HE, Arria A, et al. Unintended pregnancy in opioid-abusing women. J Subst Abuse Treat. 2011;40(2):199-202.
Rossen LM, Hamilton BE, Abma JC, et al. Updated methodology to estimate overall and unintended pregnancy rates in the United States. Vital and health statistics. Series 2, Data evaluation and methods research. 2023; 201. [Internet]. https://stacks.cdc.gov/view/cdc/124395. Accessed 24 Aug 2023
Fischbein RL, Lanese BG, Falletta L, Hamilton K, King JA, Kenne DR. Pregnant or recently pregnant opioid users: contraception decisions, perceptions and preferences. Contracept Reprod Med. 2018;3:4.
Collier KW, MacAfee LK, Kenny BM, Meyer MC. Does co-location of medication assisted treatment and prenatal care for women with opioid use disorder increase pregnancy planning, length of interpregnancy interval, and postpartum contraceptive uptake? J Subst Abuse Treat. 2019;98:73-7.
Smith C, Morse E, Busby S. Barriers to Reproductive Healthcare for Women With Opioid Use Disorder. J Perinat Neonatal Nurs. 2019;33(2):E3-e11.
Corsi DJ, Murphy MSQ. The Effects of opioids on female fertility, pregnancy and the breastfeeding mother-infant dyad: A Review. Basic Clin Pharmacol. 2021;128(5):635-41.
Daniell HW. Opioid endocrinopathy in women consuming prescribed sustained-action opioids for control of nonmalignant pain. J Pain. 2008;9(1):28-36.
Rhodin A, Stridsberg M, Gordh T. Opioid Endocrinopathy: A Clinical Problem in Patients With Chronic Pain and Long-term Oral Opioid Treatment. Clin J Pain. 2010;26(5):374-80.
Abs R, Verhelst J, Maeyaert J, et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000;85(6):2215-22.
Kim CH, Garcia R, Stover J, Ritchie K, Whealton T, Ata MA. Androgen deficiency in long-term intrathecal opioid administration. Pain Physician. 2014;17(4):E543-8.
Reddy RG, Aung T, Karavitaki N, Wass JA. Opioid induced hypogonadism. Bmj. 2010;341:c4462.
Stowell MA, Thomas-Gale T, Jones HE, Binswanger I, Rinehart DJ. Perspectives among women receiving medications for opioid use disorder: Implications for development of a peer navigation intervention to improve access to family planning services. Subst Abus. 2022;43(1):722-32.
Leridon H. Studies of fertility and fecundity: comparative approaches from demography and epidemiology. C R Biol. 2007;330(4):339-46.
Li Z, You Y, Griffin N, Feng J, Shan F. Low-dose naltrexone (LDN): A promising treatment in immune-related diseases and cancer therapy. Int Immunopharmacol. 2018;61:178-84.
Wildt L, Leyendecker G, Sir-Petermann T, Waibel-Treber S. Treatment with naltrexone in hypothalamic ovarian failure: induction of ovulation and pregnancy. Hum Reprod. 1993;8(3):350-8.
Roozenburg BJ, van Dessel HJ, Evers JL, Bots RS. Successful induction of ovulation in normogonadotrophic clomiphene resistant anovulatory women by combined naltrexone and clomiphene citrate treatment. Hum Reprod. 1997;12(8):1720-2.
Genazzani AD, Petraglia F, Gastaldi M, Volpogni C, Gamba O, Genazzani AR. Naltrexone treatment restores menstrual cycles in patients with weight loss-related amenorrhea. Fertil Steril. 1995;64(5):951-6.
Ahmed MI, Duleba AJ, El Shahat O, Ibrahim ME, Salem A. Naltrexone treatment in clomiphene resistant women with polycystic ovary syndrome. Hum Reprod. 2008;23(11):2564-9.
Maksym RB, Hoffmann-Młodzianowska M, Skibińska M, Rabijewski M, Mackiewicz A, Kieda C. Immunology and Immunotherapy of Endometriosis. J Clin Med. 2021;10(24):5879.
Xu KY, Presnall N, Mintz CM, et al. Association of Opioid Use Disorder Treatment With Alcohol-Related Acute Events. Jama Netw Open. 2021;4(2):e210061.
Xu KY, Mintz CM, Presnall N, Bierut LJ, Grucza RA. Association of Bupropion, Naltrexone, and Opioid Agonist Treatment With Stimulant-Related Admissions Among People With Opioid Use Disorder: A Case-Crossover Analysis. J Clin Psychiat. 2022;83(4):21m14112.
Xu KY, Borodovsky JT, Presnall N, et al. Association Between Benzodiazepine or Z-Drug Prescriptions and Drug-Related Poisonings Among Patients Receiving Buprenorphine Maintenance: A Case-Crossover Analysis. Am J Psychiat. 2021;178(7):651-9.
Allison P, Christakis NA. Fixed-effects methods for the analysis of nonrepeated events. Sociol Methodol. 2006;36:155-72.
Allison P, editor. Fixed Effects Regression Methods in SAS. SUGI 31 Proceedings; Paper 184-31; 2006; San Francisco, CA. Accessed August 25, 2023. Available at https://support.sas.com/resources/papers/proceedings/proceedings/sugi31/184-31.pdf.
Saitz R, Miller SC, Fiellin DA, Rosenthal RN. Recommended Use of Terminology in Addiction Medicine. J Addict Med. 2021;15(1):3-7.
Volkow ND, Gordon JA, Koob GF. Choosing appropriate language to reduce the stigma around mental illness and substance use disorders. Neuropsychopharmacology. 2021;46(13):2230-2.
Xu KY, Jones HE, Schiff DM, et al. Initiation and treatment discontinuation of medications for opioid use disorder in pregnant compared with nonpregnant people. Obstet Gynecol. 2023;141(4):845-853.
Yoder M, Boudreaux M. The effect of contraceptive access reform on privately insured patients: evidence from delaware contraceptive access now. Plos One. 2023;18(1):e0280588.
Johansson BA, Berglund M, Lindgren A. Efficacy of maintenance treatment with naltrexone for opioid dependence: a meta-analytical review. Addiction (Abingdon, England). 2006;101(4):491-503.
Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377(9776):1506-13.
World Health Organization Department of Reproductive Health and Research (WHO/RHR) and Johns Hopkins Bloomberg School of Public Health/Center for Communication Programs (CCP). Knowledge for Health Project. Family Planning: A Global Handbook for Providers (2018 update). Baltimore and Geneva: CCP and WHO; 2018. Available from: https://apps.who.int/iris/bitstream/handle/10665/260156/9780999203705-eng.pdf?sequence=1. Accessed 25 Aug 2023
Bello JK, Baxley C, Weinstock J. Preconception Health Services for Women with Opioid Use Disorder (OUD): A Review and Best Practice Recommendation. Transl Issues Psychol Sci. 2021;7(2):154-65.
Bornstein M, Gipson JD, Bleck R, Sridhar A, Berger A. Perceptions of Pregnancy and Contraceptive Use: An In-Depth Study of Women in Los Angeles Methadone Clinics. Womens Health Issues. 2019;29(2):176-81.
MacAfee LK, Dalton V, Terplan M. Pregnancy Intention, Risk Perception, and Contraceptive Use in Pregnant Women Who Use Drugs. J Addict Med. 2019;13(3):177-81.
Gilliam ML, Neustadt A, Gordon R. A call to incorporate a reproductive justice agenda into reproductive health clinical practice and policy. Contraception. 2009;79(4):243-6.
Matusiewicz AK, Melbostad HS, Heil SH. Knowledge of and concerns about long-acting reversible contraception among women in medication-assisted treatment for opioid use disorder. Contraception. 2017;96(5):365-9.
Mintz CM, Xu KY, Presnall NJ, et al. Analysis of Stimulant Prescriptions and Drug-Related Poisoning Risk Among Persons Receiving Buprenorphine Treatment for Opioid Use Disorder. JAMA Netw Open. 2022;5(5):e2211634.
Schiff DM, Work EC, Foley B, et al. Perinatal opioid use disorder research, race, and racism: a scoping review. Pediatrics. 2022;149(3):e2021052368.
Funding
This project was funded by R21 DA044744 (PI: Richard Grucza/Laura Bierut). Effort for some personnel was supported by grants K23 DA053433 (Jennifer K. Bello) and T32 DA015035 (Kevin Xu, PI: Kathleen Bucholz, Jeremy Goldbach) and St. Louis University Research Institute Fellowship (Richard Grucza), but these grants did not fund the analyses of the Merative™ MarketScan® Commercial and Multi-State Medicaid Database data performed by Dr. Xu. In addition, we acknowledge Matt Keller MS, John Sahrmann MS, Dustin Stwalley MA, and the Center for Administrative Data Research (CADR) at Washington University for assistance with data acquisition, management, and storage. Merative and MarketScan are trademarks of Merative Corporation in the US, other countries, or both. CADR is supported in part by the Washington University Institute of Clinical and Translational Sciences via grants UL1 TR002345 (from the National Center for Advancing Translational Sciences of the National Institutes of Health).
Author information
Authors and Affiliations
Contributions
Patricia Cavazos-Rehg PhD and Laura Bierut MD of the NIDA K12 Program of Washington University for obtaining funding to support effort for personnel (Dr. Xu); Matthew Keller MS, John Sahrmann MS, Dustin Stwalley MA from the Center for Administrative Data Research (CADR) of Washington University for technical support of the MarketScan Databases. Merative and MarketScan are trademarks of Merative Corporation in the US, other countries, or both.
Corresponding author
Ethics declarations
Conflict of Interest:
Dr Grucza reported receiving grants from the NIH and Arnold Ventures LLC during the conduct of the study, consulting for Janssen Pharmaceuticals, and receiving personal fees for grant reviews from the NIH and Washington University outside the submitted work. Dr. Bello reported receiving personal fees for grant reviews from the NIH outside of the submitted work.
Additional information
Prior Presentations
Bello JK, Xu K, Grucza R. (May 2023) Association between Medications for Opioid Use Disorder (MOUD) and Pregnancy. 5th Annual Institute of Clinical and Translational Sciences Symposium and Poster Display. Washington University in Saint Louis, MO. Poster presentation.
Bello, JK, Xu K, Grucza R. (September 2023) Using Big Data to Optimize Reproductive Health in Pregnant Women with Opioid Use Disorder. Institute of Clinical and Translational Sciences Big Data Research Symposium. The Advanced Health Data Institute, Saint Louis University and the Administrative Data Core Services, Washington University in Saint. Louis, MO. Oral presentation.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bello, J., Xu, K., Salas, J. et al. Pregnancy Rates Among Women Treated with Medication for Opioid Use Disorder. J GEN INTERN MED (2024). https://doi.org/10.1007/s11606-024-08689-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11606-024-08689-8