Abstract
Objective
The aim of this study was to evaluate the effectiveness of a digital health intervention plus community health worker (CHW) support on self-monitoring of blood glucose and glycosylated hemoglobin (HbA1c) among adult Medicaid beneficiaries with diabetes.
Design
Randomized controlled trial. Setting: Urban outpatient clinic.
Participants
Adult Medicaid beneficiaries living with diabetes and treated with insulin and who had a HbA1c ≥ 9%.
Intervention
Participants were randomly assigned to one of three arms. Participants in the usual-care arm received a wireless glucometer if needed. Those in the digital arm received a lottery incentive for daily glucose monitoring. Those in the hybrid arm received the lottery plus support from a CHW if they had low adherence or high blood glucose levels.
Main Measures
The primary outcome was the difference in adherence to daily glucose self-monitoring at 3 months between the hybrid and usual-care arms. The secondary outcome was difference in HbA1c from baseline at 6 months.
Key Results
A total of 150 participants were enrolled in the study. A total of 102 participants (68%) completed the study. At 3 months, glucose self-monitoring rates in the hybrid versus usual-care arms were 0.72 vs 0.65, p = 0.23. At 6 months, change in HbA1c in the hybrid versus usual-care arms was − 0.74% vs − 0.49%, p = 0.69.
Conclusion
There were no statistically significant differences between the hybrid and usual care in glucose self-monitoring adherence or improvements in HbA1C.
Trial Registration
This trial is registered with clinicaltrials.gov identifier: NCT03939793.
Similar content being viewed by others
Introduction
In the USA, over 34.2 million people have diabetes.1 Diabetes disproportionately affects people facing health and social inequity, who are often insured by Medicaid. Black, Latinx, and American Indian/Alaska Native individuals experience higher rates of diabetes and diabetes-related complications than non-Latinx white individuals,1,2 and individuals insured by Medicaid are 10% more likely to develop diabetes compared to those on Medicare.3 Managing diabetes requires complex self-management behaviors, which may be difficult for those experiencing socioeconomic barriers such as food insecurity, housing instability, unemployment, or structural discrimination within healthcare.4,5
Two intervention strategies have shown promise for promoting behavior change and improving health outcomes in a variety of populations: digital health interventions and community health workers (CHWs). Digital health interventions use computers or mobile devices to encourage self-monitoring of health metrics such as blood glucose. By raising an individual’s awareness of their metrics and health risks, digital health interventions can shift attitudes to promote healthy behavior. Digital interventions can be augmented by behavioral economic engagement strategies such as lottery-based financial incentives, which reinforce self-monitoring behavior and foster habit formation. Digital health interventions have significantly increased physical activity, improved glycemic management and smoking cessation rates, and helped people lose weight.6,7 Yet, digital health interventions have low uptake and high attrition.7 People with lower incomes are less likely to have a smartphone or home broadband internet.8 Since digital health interventions do not address underlying socioeconomic barriers that lead to poor health outcomes, people may find it self-defeating to monitor health metrics without support to improve them, contributing to high attrition.
CHWs are public health workers who have a close understanding of a community and/or are trusted members of that community.9 CHWs support patients in developing and sustaining health-promoting behaviors by shifting social norms and attitudes,10 improving self-efficacy,11,12 and addressing socioeconomic barriers by facilitating connections to resources.13 CHW interventions have a record of improving diabetes outcomes.14,15,16,17 However, CHW interventions are resource-intensive and potentially less scalable than digital health interventions. Some patients can also become discouraged and ashamed by failed attempts at behavior change, and disengage from their CHW.18 Two behavioral techniques, positive affect induction and attribution retraining, may help individuals cope with failed attempts at change. Positive affect induction uses self- affirmation and “random acts of kindness” to bolster emotional resilience after failure. Attribution retraining teaches individuals to interpret failure as resulting from controllable concrete causes rather than from character flaws.19,20,21,22,23 Although CHWs are well-suited to delivering these resilience interventions as part of the person-centered support they provide, to our knowledge, this has not yet been done.
In this study, we combined a digital health intervention designed to promote self- monitoring of blood glucose (SMBG) with a CHW intervention that included strategies for coping with setbacks and failure. We tested this hybrid intervention among adult Medicaid beneficiaries with insulin-dependent diabetes.
Methods Study Design
This study was a three-arm, type 1 effectiveness-implementation trial that combined a single-blind randomized controlled trial with qualitative process interviews. Detailed methods have been previously described.24 The study was approved by the Institutional Review Board of the University of Pennsylvania.
Setting and Participants
Participants were recruited from May 22 through December 19, 2019. Eligible participants were patients from an urban academic outpatient facility and met the following criteria: (1) diagnosed with diabetes mellitus based on ICD-10-CM codes documented within the year prior to enrollment, (2) hemoglobin A1c (HbA1c) level ≥ 9% within the prior 6 months, (3) insulin-dependent and advised to perform daily SMBG,25 (4) aged ≥ 18 years, and (5) lived in a high-poverty zip code in Philadelphia, PA. Exclusion criteria included use of a continuous glucose monitor, in another study that involved SMBG, already working with an IMPaCT CHW, or unable/unwilling to consent.
Study Procedures
After obtaining written consent, research assistants collected baseline clinical, psychosocial, demographic, and psychometric data, including SF-12,26 Adverse Childhood Experiences,27 Single-Item Drug Screen,28 Single-Item Alcohol Screen,29 Single-Item Health Literacy Screen,30 the Perceived Stress Scale,31 Enriched Social Support Inventory,32 and Patient Activation Measure.33 Research assistants used a script and low-literacy visual aid to share participants’ current HbA1c, provide brief education around evidence-based behavioral strategies for improving diabetes outcomes, and set a realistic goal for reducing HbA1c over the 6- month study period. Baseline HbA1c were collected onsite for participants who did not already have a HbA1c recorded within 28 days prior to enrollment.
If participants did not have a glucometer or if their glucometer was incompatible with the data management platform used by the study team, they were provided with a glucometer (OneTouch Verio Flex®, LifeScan Scotland Ltd.) and a 24-week supply of test strips and lancets.
Participants were asked to bring their glucometers for SMBG data extraction using the Glooko® patient management software at 3- and 6-month follow-up visits. The 3-month visit consisted of a brief assessment of adverse medical events and extraction of patients’ SMBG data including dates, times, and values since enrollment. The 6-month visit included a patient-reported outcomes survey, SMBG data extraction, adverse event screening, and HbA1c. If the participant did not attend their 6-month visit, HbA1c was extracted from the electronic health record (EHR) at 6 months ± 28 days post-baseline.
Participants received a $50 reloadable gift card upon completion of the enrollment visit, $50 upon completion of the 3-month follow-up, and $100 upon completion of the 6- month follow-up.
On March 13, 2020, the COVID-19 pandemic was declared a national emergency; at this point, 17 (11.3%) participants were eligible for but had not completed their 3-month visit, and 97 (64.7%) were eligible for but had not completed their 6-month visit. To minimize risk of harm to participants and research personnel in attending in-person visits, the study team planned several modifications that were approved by the Institutional Review Board. Remaining in-person visits were suspended and converted to telephonic visits; as a result, participants did not bring in their glucometers or have study-related labs drawn. To maximize available outcome data, the primary endpoint was shifted from 6-month to 3-month SMBG adherence, and laboratory orders for HbA1c were placed in the EHR so participants could have HbA1c drawn if they were obtaining other labs associated with their routine care.
Randomization
At enrollment, research assistants created participant profiles in the Way to Health digital health platform, an automated technology platform that integrates clinical trial randomization, biometric devices, financial system fulfillment, and secure data capture.34 Way to Health used an allocation sequence to randomize participants in a 1:1:1 ratio to one of three arms: usual care, digital health intervention, or hybrid.
Interventions
Usual Care
Participants randomized to the usual-care arm were asked to check their daily blood glucose and to continue with their usual care.
Digital Health
Participants randomized to the digital health arm were asked to check their blood glucose and text the value daily to a phone number linked to the Way to Health platform. To promote early motivation and habit formation, participants were entered into a lottery each day they texted their glucose values for the first 6 weeks of the study period. Each day participants texted, the lottery provided an 18 in 100 chance of winning $5 and 1 in 100 chance of winning $50. Accrued money was distributed to participants biweekly on a reloadable card.
If participants texted in a blood glucose value that was pre-established as medically dangerous (< 60, > 400), they received an automated text encouraging them to follow up with their provider. These values were also routed directly to the study clinician who called each patient within 24 hours to provide clinical management and coordinate care with the patient’s provider.
Hybrid Digital Health and Community Health Worker Support
Participants in the hybrid arm received all aspects of the digital health intervention: they were asked to check their blood glucose daily, were entered into a daily lottery each day they texted their blood glucose levels, and had out-of-range values routed to the study clinician who intervened as above. Additionally, CHWs met with participants at enrollment and provided brief coaching using positive affect induction and attribution retraining to increase resilience to setbacks. Details of this coaching are described elsewhere.24 At this initial meeting, CHWs explained to patients that they might work with them in the future if they needed additional support.
During the first 12 weeks of the study, hybrid arm participants with low rates of SMBG (defined as 5 instances of missed readings) and/or elevated glucose readings (defined as a glucose level > 300 mg/dL for > 30% of days over any 2-week period) were ‘escalated’ to receive intensive CHW support.
CHWs implemented the IMPaCT intervention,14,15,35 in which the CHW used an in-depth semi-structured interview guide to get to know participants’ strengths, goals, and unmet social needs (e.g., food insecurity, housing instability, drug and alcohol use, family stress, etc.). As a part of this conversation, CHWs asked: “What do you think you’ll need in order to improve your health?” Participants’ individualized goals became the basis for tailored action plans. For the remainder of the 24-week study period, CHWs provided coaching, social support, advocacy, and navigation to support participants in achieving their health goals. CHWs communicated with participants at least once per week, including monthly face-to-face contact. During these encounters, CHWs normalized setbacks and used positive affect induction and attribution retraining to help patients to cope with failure. CHWs did not provide diabetes education; however, they confirmed participant access to a glucometer and asked about their blood glucose readings. CHWs also helped participants connect to long-term family and social supports after the intervention ended.
Initially, CHWs worked with participants both by phone and in person, depending on participant preference and situation. When COVID-19–related restrictions began in March 2020, all CHW engagement transitioned to telephonic and text message support. This occurred in month 10 of the study and affected half of the participants who received CHW support. Participants who completed the intervention prior to the COVID-19 restrictions had an average of 14.3 successful contacts with the CHW, compared with an average of 10.8 contacts for participants whose intervention period overlapped with the stay-at-home order.
Outcomes
The primary outcome of interest was the difference between hybrid and usual-care arms in adherence to SMBG at 3 months, as measured by the total number of days the glucometer was used divided by the 90 days in the follow-up period. The primary outcome was measured by extracting SMBG data directly from glucometers. The secondary outcome was the difference between hybrid and usual-care arms in change in HbA1c from baseline to 6-month follow-up. As an exploratory analysis, we compared the outcomes above between hybrid and digital health arms.
Completers
We defined participants with complete adherence data to include anyone whose glucometer data included a reading before and after the primary endpoint (90 days). To avoid underestimating adherence rates, we considered participants who were lost to follow-up, who did not bring glucometers to follow-up visits, whose glucometer could not be read by the data management platform, or whose glucometer readings started after 90 days post-enrollment to be non-completers, i.e., to have missing adherence data rather than 0% adherence.
Statistical Analysis
Means, medians, standard deviations, and proportions were calculated to describe the sample. Descriptive comparisons of baseline characteristics between arms used chi-square test for categorical and Wilcoxon rank-sum test for continuous variables. We used Wilcoxon rank-sum test to assess differences between two arms for the primary and secondary outcomes.
We first conducted a complete case analysis using Poisson regression to model rate of adherence to SMBG at 3 months. We also imputed missing data, using multiple imputation with the chained equations method based on baseline characteristics. Each of the 20 imputed datasets were analyzed with a Poisson model, and results were combined for inference using Rubin’s rules. Results from two separate analyses (complete case and imputed) were nearly identical; since the data may not be missing at random, we report complete case results from the Wilcoxon rank-sum test. All statistical analyses were carried out with SAS (version 9.4). A significance level of 0.05 was used.
Sample Size
Based on a similar 24-week trial,36 we assumed that SMBG adherence rate in the usual-care arm would be 40%. We hypothesized that rate in the hybrid intervention would be 80%. Using a two-sample comparison of proportions (usual care versus hybrid), we estimated a sample size of 28 participants per arm to detect differences with 80% power, assuming a type I error rate of 0.05 and 20% attrition. We enrolled 50 participants per arm to ensure that we would exceed minimum threshold for appropriate sample size
Results
Participants
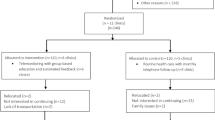
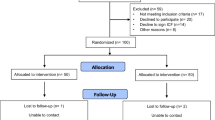
Out of 485 patients screened, 59 (12.2%) did not meet eligibility criteria. Of the remaining 426 patients, 181 (65.6%) were not interested, 79 (28.6%) did not arrive for their initial visit, and 16 (5.8%) declined to consent. A total of 150 participants were enrolled and randomized to one of three arms. Loss to follow-up was greater than anticipated at both 3 and 6 months. A total of 40 (80.0%) control arm, 34 (68.0%) digital health alone, and 28 (56.0%) hybrid participants completed data collection for the primary outcome (Fig. 1). We compared study participants who completed 3 months of SMBG adherence (N = 102) with those who did not (N = 48). The non-completers differed significantly on arm assignment (20.0% usual care vs. 33.3% digital vs. 45.4% hybrid, p = 0.04)
Participant baseline characteristics are provided in Table 199.0% of participants were insured by Medicaid (including 40.7% Medicare-Medicaid dually eligible). Participants’ mean age was 55.1 years, 76.0% had completed high school or attended college,70.0% were female, and 87.3% were Black. Participants had many socioeconomic stressors, with 62.7% reporting annual incomeof < $15,000/year and 82.7% being unemployed. Mean HbA1c at baseline was 10.96. There were no statistically significantdifferences in baseline characteristics between arms.
Outcomes
Overall, 41 hybrid arm participants (82.0%) required escalation to intensive support from a CHW because they demonstrated low adherence or elevated blood glucose levels.
At 3 months, SMBG rates in the hybrid versus usual-care arms were 0.72 vs 0.65 (p = 0.23). At 6 months, changes in HbA1c in the hybrid versus usual-care arms were − 0.74% vs − 0.49% (p = 0.69). In our exploratory analysis comparing hybrid versus digital arms, we found SMBG rates at 3 months were 0.72 vs. 0.70 (p = 0.51) and changes in HbA1c at 6 months were − 0.74% vs. − 0.85% (p = 0.36) (Table 2
Discussion
In this randomized controlled trial, which enrolled Medicaid-insured patients with insulin-dependent diabetes, we did not observe statistically significant differences in either SMBG adherence or improvement in HbA1c between any of the three arms (usual care, digital health alone, or hybrid digital health with CHW support for those with persistent elevated glucose levels or non-adherence to SMBG). All three arms received proven effective strategies for improving blood glucose management, including basic diabetes education with goal setting, which has been shown to improve HbA1c.37 Although both financial incentives and CHW support have previously been shown to be cost-effective strategies38,39 that improve diabetes outcomes14,15,35 but have not been studied in combination. Over 80% of participants in the hybrid arm demonstrated low adherence or high blood glucose levels, even with the digital health intervention, and were thus “escalated” to receive CHW support. CHWs reinforced participants’ established goals and the importance of self-monitoring of blood glucose, addressed the socio-behavioral determinants of health, and provided strategies for coping with the setbacks that individuals may encounter when living with diabetes. Interestingly, we observed greater attrition at the 3-month follow-up in the hybrid arm (44%) than in either of the other arms. This rate is higher than what we have seen in previous studies of the IMPaCT model.14,15,35 Several factors may have contributed to this attrition. Since participants knew they were only receiving CHW support because they had not succeeded in following their self-monitoring regimen, it is possible that feelings of embarrassment may have led them to disengage, consistent with a previous qualitative study.18 In addition, priming participants to expect a light-touch, technology-focused intervention, and delaying the more intensive and interactive CHW support until they struggled may also have led participants to disengage out of perceived inconvenience.
This study is timely because the COVID pandemic has catalyzed a shift toward digital health, as well as home and community-based care for conditions like diabetes. Digital health has grown as a share of health care services,40 supported by new policy flexibilities from legislators, regulators, and payers.41 In addition, a U.S. congressional bill introduced in the House of Representatives in early 2021 would invest in expanding home and community-based care through increasing the applicable Medicaid federal matching rate for those services.42 In the past, digital health interventions and community-based supports have been viewed as separate and distinct. In theory, patients would benefit from habit formation encouraged by digital health interventions combined with support to address underlying social needs. Our study demonstrates the feasibility of a hybrid approach which may overcome limitations of digital health or CHW support alone. However, the lack of improvement for the hybrid arm relative to the usual-care and digital health arms in either SMBG adherence or HbA1c, as well as the high attrition rate we observed in the hybrid arm, indicate that more research is needed to identify optimal combinations of these strategies.
Our study has some limitations. This single-center study had a relatively small sample size with high loss to follow-up limiting power and generalizability. The COVID pandemic altered our study design and may have led to a higher-than-expected rate of loss to follow-up at 6 months. Unlike many digital health interventions which require smartphones or broadband connection, we only required participants to have a basic cell phone with SMS messaging; this was an intentional modification to make the intervention more accessible to low-income populations. However, it meant that participants needed to complete an additional step of texting their SMBG to the study platform which may have contributed to increased rates of attrition in the two arms that required this step. In addition, because we used low-tech glucometers covered by Medicaid instead of ones that upload real-time data via Bluetooth connection, we were dependent on extracting data from the physical device; if someone lost or did not bring in their glucometer, this resulted in missing data. Finally, one of the COVID-19 modifications included a truncation of our primary measure (adherence) from 6 to 3 months. Future studies should include longer follow-up times.
Conclusion
In a randomized trial of Medicaid-insured patients with insulin-dependent diabetes using two behavioral interventions (digital incentives and CHW) compared with usual care, we did not find statistically significant improvements in SMBG and HbA1c. Additional studies are needed to identify the ideal sequencing, framing, and combination of digital health and CHW interventions.
References
CDC. National Diabetes Statistics Report, 2020. Centers for Disease Control and Prevention. Published February 11, 2020. Accessed February 28, 2020. https://www.cdc.gov/diabetes/library/features/diabetes-stat-report.html
Carter JS, Pugh JA, Monterrosa A. Non-insulin-dependent diabetes mellitus in minorities in the United States. Ann Intern Med. 1996;125(3):221-232. https://doi.org/10.7326/0003-4819-125-3-199608010-00011
Dinca-Panaitescu M, Dinca-Panaitescu S, Raphael D, Bryant T, Pilkington B, Daiski I. The dynamics of the relationship between diabetes incidence and low income: longitudinal results from Canada’s National Population Health Survey. Maturitas. 2012;72(3):229-235. https://doi.org/10.1016/j.maturitas.2012.03.017
Association Diabetes Association. 5. Lifestyle management: standards of medical care in diabetes—2019. Diab Care. 2019;42(Supplement 1):S46-S60. https://doi.org/10.2337/dc19-S005
CMS. Racial and ethnic disparities in diabetes prevalence, self-management, and health outcomes among Medicare. Accessed November 15, 2021. https://www.cms.gov/About-CMS/Agency-Information/OMH/research-and-data/information-products/data-highlights/disparities-in-diabetes-prevalence#:~:text=Beneficiaries%20eligible%20for%20both%20Medicare,only%20beneficiaries%20(20.2%20percent).
Pal K, Dack C, Ross J, et al. Digital health interventions for adults with type 2 diabetes: qualitative study of patient perspectives on diabetes self-management education and support. J Med Internet Res. 2018;20(2):e40. https://doi.org/10.2196/jmir.8439
Alkhaldi G, Hamilton FL, Lau R, Webster R, Michie S, Murray E. The effectiveness of prompts to promote engagement with digital interventions: a systematic review. J Med Internet Res. 2016;18(1):e6. https://doi.org/10.2196/jmir.4790
Vogels EA. Digital divide persists even as Americans with lower incomes make gains in tech option. Pew Research Center. 2021. https://pewrsr.ch/2TRM7cP. Accessed 20 Dec 2021.
American Public Health Association. Community Health Workers [Internet]. Washington DC; 2021. Accessed April 23, 2021. Available from: https://www.apha.org/apha-communities/member-sections/community-health-workers. American Public Health Association. Community Health Workers.
Kangovi S, Grande D, Carter T, et al. The use of participatory action research to design a patient-centered community health worker care transitions intervention. Healthc (Amst). 2014;2(2):136-144. https://doi.org/10.1016/j.hjdsi.2014.02.001
Wood JV. Theory and research concerning social comparisons of personal attributes. Psychol Bull. 1989; 106(2):231-248.
Simoni JM, Franks JC, Lehavot K, Yard SS. Peer interventions to promote health: conceptual considerations. Am J Orthopsychiatry. 2011;81(3):351-359. https://doi.org/10.1111/j.1939-0025.2011.01103.x
Bedell P, Wilson JL, White AM, Morse DS. “Our commonality is our past:” a qualitative analysis of re-entry community health workers’ meaningful experiences. Health Justice. 2015;3:19. https://doi.org/10.1186/s40352-015-0031-5
Kangovi S, Mitra N, Grande D, Huo H, Smith RA, Long JA. Community health worker support for disadvantaged patients with multiple chronic diseases: a randomized clinical trial. Am J Public Health. 2017;107(10):1660-1667. https://doi.org/10.2105/AJPH.2017.303985
Kangovi S, Mitra N, Norton L, et al. Effect of community health worker support on clinical outcomes of low-income patients across primary care facilities: a randomized clinical trial. JAMA Intern Med. 2018;178(12):1635-1643. https://doi.org/10.1001/jamainternmed.2018.4630
Palmas W, March D, Darakjy S, et al. Community health worker interventions to improve glycemic control in people with diabetes: a systematic review and meta-analysis. J Gen Intern Med. 2015;30(7):1004-1012. https://doi.org/10.1007/s11606-015-3247-0
Egbujie BA, Delobelle PA, Levitt N, Puoane T, Sanders D, van Wyk B. Role of community health workers in type 2 diabetes mellitus self-management: A scoping review. PLoS One. 2018;13(6):e0198424. https://doi.org/10.1371/journal.pone.0198424
Edlind M, Mitra N, Grande D, et al. Why effective interventions don’t work for all patients: exploring variation in response to a chronic disease management intervention. Med Care. 2018;56(8):719-726. https://doi.org/10.1097/MLR.0000000000000939
Peterson JC, Charlson ME, Hoffman Z, et al. Randomized controlled trial of positive affect induction to promote physical activity after percutaneous coronary intervention. Arch Intern Med. 2012;172(4):329-336. https://doi.org/10.1001/archinternmed.2011.1311
Jackson SE, Hall NC, Rowe PM, Daniels LM. Getting the job: attributional retraining and the employment interview. J Appl Soc Psych. 2009;39(4):973-998. https://doi.org/10.1111/j.1559-1816.2009.00468.x
Ilies R, Judge TA, Wagner DT. The influence of cognitive and affective reactions to feedback on subsequent goals: role of behavioral inhibition/activation. European Psychologist. 2010;15(2):121-131. https://doi.org/10.1027/1016-9040/a000011
Hall NC, Hladkyj S, Perry RP, Ruthig JC. The role of attributional retraining and elaborative learning in college students’ academic development. J Soc Psychol. 2004;144(6):591-612. https://doi.org/10.3200/SOCP.144.6.591-612
Holschuh JP, Nist SL, Olejnik S. Attributions to failure: the effects of effort, ability and learning strategy use on perceptions of future goals and emotional responses. Reading Psychology, 22:3, 153-173, https://doi.org/10.1080/027027101753170601
Harte R, Norton L, Whitehouse C, et al. Design of a randomized controlled trial of digital health and community health worker support for diabetes management among low-income patients. Contemp Clin Trials Commun. 2022;25:100878. https://doi.org/10.1016/j.conctc.2021.100878
American Diabetes Association. 7. Diabetes technology: standards of medical care in diabetes—2021. Diab Care. 2020;44(Supplement_1):S85-S99. https://doi.org/10.2337/dc21-S007
Maruish ME, Kosinski M. A Guide to the Development of Certified Short Form Survey Interpretation and Reporting Capabilities. QualityMetric, Incorporated; 2009.
Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The adverse childhood experiences (ACE) study. Am J of Prev Med. 1998;14(4):245-258. https://doi.org/10.1016/S0749-3797(98)00017-8
Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170(13):1155-1160. https://doi.org/10.1001/archinternmed.2010.140
Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R. Primary care validation of a single-question alcohol screening test. J Gen Inter Med. 2009;24(7):783-788. https://doi.org/10.1007/s11606-009-0928-6
Morris NS, MacLean CD, Chew LD, Littenberg B. The single item literacy screener: evaluation of a brief instrument to identify limited reading ability. BMC Fam Pract. 2006;7,21. https://doi.org/10.1186/1471-2296-7-21
Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385-396. https://doi.org/10.2307/2136404
Mitchell PH, Powell L, Blumenthal J, et al. A short social support measure for patients recovering from myocardial infarction: the ENRICHD Social Support Inventory. J Cardiopulm Rehabil. 2003;23(6):398-403. https://doi.org/10.1097/00008483-200311000-00001
Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39(4 Pt 1):1005-1026. https://doi.org/10.1111/j.1475-6773.2004.00269.x
Asch DA, Volpp KG. On the Way to Health. LDI Issue Brief. 2012;17(9):1-4.
Kangovi S, Mitra N, Grande D, et al. Patient-centered community health worker intervention to improve posthospital outcomes: a randomized clinical trial. JAMA Intern Med. 2014;174(4):535-543. https://doi.org/10.1001/jamainternmed.2013.14327
Sen AP, Sewell TB, Riley EB, et al. Financial incentives for home-based health monitoring: a randomized controlled trial. J Gen Inter Med. 2014;29(5):770-777. https://doi.org/10.1007/s11606-014-2778-0
Kocak MZ, Aktas G, Erkus E, Duman TT, Atak BM, Savli H. Analysis of the type 2 diabetic patients followed in a University clinic. Konuralp Medical Journal. 2018;10(2):198-202. https://doi.org/10.18521/ktd.345149
Egede LE, Walker RJ, Dismuke-Greer CE, et al. Cost-effectiveness of financial incentives to improve glycemic control in adults with diabetes: A pilot randomized controlled trial. PLOS ONE. 2021;16(3):e0248762. https://doi.org/10.1371/journal.pone.0248762
Kangovi S, Mitra N, Grande D, Long JA, Asch DA. Evidence-based community health worker program addresses unmet social needs and generates positive return on investment. Health Affairs. 2020;39(2):207-213. https://doi.org/10.1377/hlthaff.2019.00981
Mehrotra A, Chernew ME, Linetsky D, et al. The impact of COVID-19 on outpatient visits in 2020: visits remained stable, despite a late surge in cases. Commonwealth Fund. Feb. 2021. https://doi.org/10.26099/bvhf-e411
Manatt. Executive Summary: Tracking Telehealth Changes State-by-State in Response to COVID-19. Accessed November 15, 2021. https://manatt.com/insights/newsletters/covid-19-update/executive-summary-tracking-telehealth-changes-stat
Dingell D. Text - H.R.525 - 117th Congress (2021-2022): COVID HCBS Relief Act of 2021. Published February 2, 2021. Accessed November 15, 2021. https://www.congress.gov/bill/117th-congress/house-bill/525/text
Funding
This work is supported by a grant from the Commonwealth Fund (#20191985) and by grant K23-HL128837 from the National Institutes of Health National Heart, Lung, and Blood Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Whitehouse, C.R., Knowles, M., Long, J.A. et al. Digital Health and Community Health Worker Support for Diabetes Management: a Randomized Controlled Trial. J GEN INTERN MED 38, 131–137 (2023). https://doi.org/10.1007/s11606-022-07639-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-022-07639-6