Abstract
Background
Gastric cancer is the fifth most common malignancy and the fourth most common cause of cancer mortality globally. The role of neoadjuvant chemotherapy in upfront resectable gastric cancer is a subject of ongoing research. In recent meta-analyses, R0 resection rate and superior outcomes were not consistently observed in such regimens.
Aim
To describe the outcomes following phase III randomised control trials; comparing neoadjuvant therapy followed by surgery against upfront surgery with and without adjuvant therapy in resectable gastric cancers.
Methods
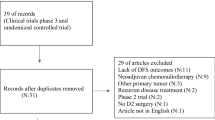
The Cochrane Library, CINAHL, EMBASE, PubMed, SCOPUS and Web of Science was searched from January 2002 to September 2022.
Results
13 studies were included (3280 participants). R0 resection rates were in neoadjuvant therapy arms as compared to adjuvant therapy with odds ratio (OR) 1.55[95% CI: 1.13, 2.13](p=0.007) and compared to surgery alone OR 2.49[95% CI: 1.56, 3.96](p=0.0001). 3-year and 5-year progression-, event- and disease-free survival in neoadjuvant therapy as compared to adjuvant therapy were not significantly increased, 3-year OR 0.87[0.71, 1.07](p=0.19). Meanwhile, comparing neoadjuvant therapy to adjuvant therapy, 3-year overall survival (OS) hazard ratio was 0.88[95% CI: 0.70, 1.11](p=0.71) while 3- and 5-year OS OR was 1.18[95% CI: 0.90, 1.55], p=0.22 and 1.27[95% CI: 0.67, 2.42](p=0.47) respectively. Surgical complications were also more common with neoadjuvant therapy.
Conclusion
Neoadjuvant therapy yields higher rates of R0 resection. However, improved long-term survival was not seen as compared to adjuvant therapy. Large multi-centred randomised control trials with D2 lymphadenectomy should be performed to better evaluate the treatment modalities.






Similar content being viewed by others
References
Takahashi T, Saikawa Y, Kitagawa Y. Gastric Cancer: Current Status of Diagnosis and Treatment. Cancers (Basel). 2013;5:48–63.
Sitarz R, Skierucha M, Mielko J, Offerhaus JA, Maciejewski R, Polkowski WP. Cancer Management and Research Dovepress Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10 239.
Coccolini F, Montori G, Ceresoli M, Nita GE, Ansaloni L, Cima S, et al. Advanced gastric cancer: what we know and what we still have to learn peritoneal; Surgery; Definition. World J Gastroenterol. 2016;22:1139–59.
Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23:7114–24.
Johnston FM, Beckman M. Updates on Management of Gastric Cancer. Curr Oncol Rep. 2019;21:67.
Proserpio I, Rausei S, Barzaghi S, Frattini F, Galli F, Iovino D, et al. Multimodal treatment of gastric cancer. World J Gastrointest Surg. 2014;6:55–8.
Webb A, Cunningham D, Scarffe JH, Harper P, Norman A, Joffe JK, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997;15:261–7.
Cunningham D, Allum WH, Stenning SP, Thompson JN, van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer Engl J Med. 2006;355(1), 11-20.
Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–8.
Yu J-H, Wang Z-Z, Fan Y-C, Liu M-X, Xu K, Zhang N, et al. Comparison of neoadjuvant chemotherapy followed by surgery vs. surgery alone for locally advanced gastric cancer: a meta-analysis. Chin Med J. 2021;134(14), 1669-1680
Yu X, Hu Y, Hu D, Li W. Neoadjuvant chemotherapy brings more survival benefits than postoperative chemotherapy for resectable gastric cancer: a Meta-analysis of randomized controlled trials. JBUON. 2019;24:201–14.
Eom SS, Choi W, Eom BW, Park SH, Kim SJ, Kim YI, et al. A Comprehensive and Comparative Review of Global Gastric Cancer Treatment Guidelines. J Gastric Cancer. Korean Gastric Cancer Association; 2022;22:3.
Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun. Wiley-Blackwell; 2021;41:747.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018. Gastric Cancer 2021;24 1:121.
Ryu KW, Park YS, Kwon OK, Oh J, Lee HH, Kong SH, et al. Korean Practice Guideline for Gastric Cancer 2018: an Evidence-based, Multi-disciplinary Approach. J Gastric Cancer. Korean Gastric Cancer Association; 2019;19:1.
Okines A, Verheij M, Allum W, Cunningham D, Cervantes A. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology. Elsevier; 2010;21:v50–4.
Songun I, Putter H, Kranenbarg EMK, Sasako M, van de Velde CJH. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol.; 2010;11:439–49.
Burke DL, Billingham LJ, Girling AJ, Riley RD. Meta-analysis of randomized phase II trials to inform subsequent phase III decisions. Trials. BioMed Central Ltd.; 2014;15:1–15.
Liang F, Wu Z, Mo M, Zhou C, Shen J, Wang Z, et al. Comparison of treatment effect from randomised controlled phase II trials and subsequent phase III trials using identical regimens in the same treatment setting. Eur J Cancer. Pergamon; 2019;121:19–28.
Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, et al. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients With Locally Advanced Gastric Cancer: The CLASS-01 Randomized Clinical Trial. JAMA. American Medical Association; 2019;321:1983.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. BioMed Central; 2007;8:1–16.
Duval S, Tweedie R. A Nonparametric “Trim and Fill” Method of Accounting for Publication Bias in Meta-Analysis. J Am Stat Assoc. ; 2012;95:89–98.
Lin L, Chu H. Quantifying Publication Bias in Meta-Analysis. Biometrics.; 2018;74:785.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. BMJ; 2019;366.
Xue K, Ying X, Bu Z, Wu A, Li Z, Tang L, et al. Oxaliplatin plus S-1 or capecitabine as neoadjuvant or adjuvant chemotherapy for locally advanced gastric cancer with D2 lymphadenectomy: 5-year follow-up results of a phase II - III randomized trial. Chin J Cancer Res.; 2018;30:516–25.
Zhao Q, Lian C, Huo Z, Li M, Liu Y, Fan L, et al. The efficacy and safety of neoadjuvant chemotherapy on patients with advanced gastric cancer: A multicenter randomized clinical trial. Cancer Med.2020;9:5731–45.
Basi A, Sohrabkhani S, Zamani F, Baghai-Wadji M, Rabiei N, Razavi S-M, et al. Comparing Efficacy of Preoperative neo-Adjuvant Chemotherapy and Surgery versus Surgery Alone in Patients with Resectable Gastroesophageal Cancer. Int J Hematol Oncol Stem Cell Res. 2013;7:24–8.
Hashemzadeh S, Pourzand A, Somi MH, Zarrintan S, Javad-Rashid R, Esfahani A. The effects of neoadjuvant chemotherapy on resectability of locally-advanced gastric adenocarcinoma: a clinical trial. Int J Surg. Int J Surg; 2014;12:1061–9.
Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28:5210–8.
Fazio N, Biffi R, Maibach R, Hayoz S, Thierstein S, Brauchli P, et al. Preoperative versus postoperative docetaxel-cisplatin-fluorouracil (TCF) chemotherapy in locally advanced resectable gastric carcinoma: 10-year follow-up of the SAKK 43/99 phase III trial. Ann Oncol. Ann Oncol; 2016;27:668–73.
Biffi R, Fazio N, Luca F, Chiappa A, Andreoni B, Zampino MG, et al. Surgical outcome after docetaxel-based neoadjuvant chemotherapy in locally-advanced gastric cancer. . World J. Gastroenterol.: WJG; 2010;16:868.
Ychou M, Boige V, Pignon J-P, Conroy T, Bouché O, Lebreton G, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715–21.
Ramachandra, Goel V, Raju K, Rao TS, Patnaik, Nusrath, et al. Prospective Randomized Controlled Study Comparing Primary Surgery Versus Neoadjuvant Chemotherapy Followed by Surgery in Gastric Carcinoma. Indian J Surg Oncol. 2019;10:245–50.
Kang YK, Yook JH, Park YK, Lee JS, Kim YW, Kim JY, et al. PRODIGY: A Phase III Study of Neoadjuvant Docetaxel, Oxaliplatin, and S-1 Plus Surgery and Adjuvant S-1 Versus Surgery and Adjuvant S-1 for Resectable Advanced Gastric Cancer. J Clin Oncol. J Clin Oncol; 2021;39:2903–13.
Iwasaki Y, Terashima M, Mizusawa J, Katayama H, Nakamura K, Katai H, et al. Gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer (JCOG0501): an open-label, phase 3, randomized controlled trial. Gastric Cancer. Gastric Cancer; 2021;24:492–502.
Terashima M, Iwasaki Y, Mizusawa J, Katayama H, Nakamura K, Katai H, et al. Randomized phase III trial of gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer, the short-term safety and surgical results: Japan Clinical Oncology Group Study (JCOG0501). Gastric Cancer. Gastric Cancer; 2019;22:1044–52.
Zhang X, Liang H, Li Z, Xue Y, Wang Y, Zhou Z, et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol.; 2021;22:1081–92.
Biondi A, Persiani R, Cananzi F, Zoccali M, Vigorita V, Tufo A, et al. R0 resection in the treatment of gastric cancer: Room for improvement resection in the treatment of gastric cancer: Room for improvement. World J Gastroenterol. 2010;16:3358–70.
Matuschek C, Jazmati D, Bölke E, Tamaskovics B, Corradini S, Budach W, et al. Post-Neoadjuvant Treatment Strategies in Breast Cancer. Cancers (Basel).; 2022:14(5)1246.
Rosenblatt R, Sherif A, Rintala E, Wahlqvist R, Ullén A, Nilsson S, et al. Pathologic downstaging is a surrogate marker for efficacy and increased survival following neoadjuvant chemotherapy and radical cystectomy for muscle-invasive urothelial bladder cancer. Eur Urol. 2012;61:1229–38.
Cui H, Cui J-X, Wang Y-N, Cao B, Deng H, Zhang K-C, et al. Could neoadjuvant chemotherapy increase postoperative complication risk of laparoscopic total gastrectomy? A mono-institutional propensity score-matched study in China. World J Gastrointest Surg. 2021;13:429–42.
Imyanitov EN, Yanus GA. Neoadjuvant therapy: theoretical, biological and medical consideration. Chin Clin Oncol. Chin Clin Oncol; 2018;7.
Xiong B-H, Cheng Y, Ma L, Zhang C-Q. An updated meta-analysis of randomized controlled trial assessing the effect of neoadjuvant chemotherapy in advanced gastric cancer. Cancer Invest. 2014;32:272–84.
Xu AM, Huang L, Liu W, Gao S, Han WX, Wei ZJ. Neoadjuvant Chemotherapy Followed by Surgery versus Surgery Alone for Gastric Carcinoma: Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS One.; 2014;9:e86941.
Cai Z, Yin Y, Zhao Z, Xin C, Cai Z, Yin Y, et al. Comparative Effectiveness of Neoadjuvant Treatments for Resectable Gastroesophageal Cancer: A Network Meta-Analysis. Front Pharmacol. 2018;9:872.
Liao Y, Yang Z, Peng J, Xiang J, Wang J. Neoadjuvant chemotherapy for gastric cancer: a meta-analysis of randomized, controlled trials. J Gastroenterol Hepatol. 2013;28:777–82.
Sevdalis N, Jacklin R. Interaction effects and subgroup analyses in clinical trials: more than meets the eye? J Eval Clin Pract. 2008;14:919–22.
Wang X, Li S, Sun Y, Li K, Shen X, Xue Y, et al. The protocol of a prospective, multicenter, randomized, controlled phase III study evaluating different cycles of oxaliplatin combined with S-1 (SOX) as neoadjuvant chemotherapy for patients with locally advanced gastric cancer: RESONANCE-II trial. BMC Cancer. 2021;21:20.
Liu X, Jin J, Cai H, Huang H, Zhao G, Zhou Y, et al. Study protocol of a randomized phase III trial of comparing preoperative chemoradiation with preoperative chemotherapy in patients with locally advanced gastric cancer or esophagogastric junction adenocarcinoma: PREACT. BMC Cancer. 2019;19:606: https://doi.org/10.1186/s12885-019-5728-8
Tokunaga M, Mizusawa J, Machida N, Fukagawa T, Katai H, Nishida Y, et al. Phase III trial to evaluate the efficacy of neoadjuvant chemotherapy with S-1 plus oxaliplatin followed by D2 gastrectomy with adjuvant S-1 in locally advanced gastric cancer: Japan Clinical Oncology Group study JCOG1509 (NAGISA trial). J Clin Oncol.; 2017;35:TPS4134–TPS4134.
Tabernero J, Bang Y-J, van Cutsem E, Fuchs CS, Janjigian YY, Bhagia P, et al. KEYNOTE-859: a Phase III study of pembrolizumab plus chemotherapy in gastric/gastroesophageal junction adenocarcinoma. Future Oncol. 2021;17:2847–55.
Dindo D, Demartines N, Clavien PA. Classification of Surgical Complications: A New Proposal With Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann Surg. 2004;240:205.
Acknowledgements
This study was not funded, we would like to thank Lee Kong Chian School of Medicine’s Library for their assistance with the search strategy.
Author information
Authors and Affiliations
Contributions
Ho Si Ying, Adelina: acquisition, analysis and interpretation of data, manuscript drafting and approval, agreement for accountability for all aspects of the work
Lim Khai Shin, Alva: project development, acquisition, analysis and interpretation of data, manuscript drafting and approval, agreement for accountability for all aspects of the work
Sarah Neo Hui Wen: acquisition of data, manuscript drafting and approval, agreement for accountability for all aspects of the work
Charleen Yeo Shan Wen: project development, interpretation of data, manuscript drafting and approval, agreement for accountability for all aspects of the work
Tay Kon Voi: project development, interpretation of data, manuscript drafting and approval, agreement for accountability for all aspects of the work
Corresponding author
Ethics declarations
Registration and protocol
Review was not registered.
Conflict of interests
The authors declare no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Exempt from ethics board approval
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lim Khai Shin, A., Ho Si Ying, A., Neo Hui Wen, S. et al. Systematic review and meta-analysis of the outcomes following neoadjuvant therapy in upfront resectable gastric cancers compared to surgery alone in phase III randomised controlled trials. J Gastrointest Surg 27, 1261–1276 (2023). https://doi.org/10.1007/s11605-023-05641-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-023-05641-9