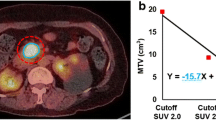
Abstract
Purpose
To evaluate the clinical significance of 18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with pancreatic ductal adenocarcinoma who underwent neoadjuvant therapy.
Methods
Among 285 consecutive patients who underwent pancreatic resection for pancreatic ductal adenocarcinoma between 2015 and 2021, 86 who underwent preoperative 18F-fluorodeoxyglucose positron emission tomography/computed tomography after completion of neoadjuvant treatment were reviewed. Among preoperative factors, including post-treatment maximum standardized uptake value, predictors of early recurrence and poor prognosis were identified using multivariate analysis for decision making in surgery.
Results
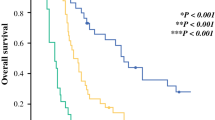
Nineteen (22%) patients with pancreatic ductal adenocarcinoma demonstrated high maximum standardized uptake (≥ 4.5). High post-treatment maximum standardized uptake (≥ 4.5) predicted early recurrence within 6 months after surgery and correlated with shorter recurrence-free survival. Elevated post-treatment CA19-9 level (> 37 U/ml) and maximum standardized uptake ≥ 4.5 were independent prognostic factors. Post-treatment, a high maximum standardized uptake value indicated a poorer prognosis than a low maximum standardized uptake value in both patients with elevated CA19-9 and normal CA19-9 levels. The median overall survival in patients with elevated post-treatment CA19-9 and high maximum standardized uptake was only 17 months; 67% experienced early recurrence. Dynamic changes in maximum standardized uptake during neoadjuvant therapy were correlated with pathological response to neoadjuvant therapy, but not with radiological response or change in CA19-9 level.
Conclusions
Post-treatment assessment using maximum standardized uptake value is useful for stratifying patients with pancreatic ductal adenocarcinoma who will benefit from surgery. Instead of subsequent curative resection, additional neoadjuvant therapy should be considered in patients with a persistently high maximum standardized uptake value.


Similar content being viewed by others
References
Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33.
Matsumoto I, Murakami Y, Shinzeki M, et al. Proposed preoperative risk factors for early recurrence in patients with resectable pancreatic ductal adenocarcinoma after surgical resection: a multi-center retrospective study. Pancreatology 2015;15:674-680.
Ono S, Adachi T, Ohtsuka T, et al. Predictive factors for early recurrence after pancreaticoduodenectomy in patients with resectable pancreatic head cancer: a multicenter retrospective study. Surgery 2022;172:1782-1790.
Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). The Lancet 2016;388:248-257.
Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389:1011-1024.
Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med 2018;379:2395-2406.
Unno M, Motoi F, Matsuyama Y. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer(Prep-02/JSAP05). J Clin Oncol 2019;37(suppl 4):abstr 189.
Versteijne E, van Dam JL, Suker M, et al. Neoadjuvant chemoradiotherapy versus upfront surgery for resectable and borderline resectable pancreatic cancer: long-term results of the Dutch randomized PREOPANC Trial. J Clin Oncol 2022;40:1220-1230.
Tempero MA, Malafa MP, Chiorean EG, et al. Pancreatic adenocarcinoma, version 1.2019. J Natl Compr Canc Netw 2019;17:202-210.
Ushida Y, Inoue Y, Ito H, et al. High CA19-9 level in resectable pancreatic cancer is a potential indication of neoadjuvant treatment. Pancreatology 2021;21:130-137.
Takahashi H, Yamada D, Asukai K, et al. Clinical implications of the serum CA19-9 level in "biological borderline resectability" and "biological downstaging" in the setting of preoperative chemoradiation therapy for pancreatic cancer. Pancreatology 2020;20:919-928.
Tsai S, George B, Wittmann D, et al. Importance of normalization of CA19-9 levels following neoadjuvant therapy in patients with localized pancreatic cancer. Ann Surg 2020;271:740-747.
Aoki S, Motoi F, Murakami Y, et al. Decreased serum carbohydrate antigen 19-9 levels after neoadjuvant therapy predict a better prognosis for patients with pancreatic adenocarcinoma: a multicenter case-control study of 240 patients. BMC Cancer 2019;19:252.
Groot VP, Mosier S, Javed AA, et al. Circulating Tumor DNA as a Clinical Test in Resected Pancreatic Cancer. Clin Cancer Res 2019;25:4973-4984.
Hillner BE, Siegel BA, Shields AF, et al. Relationship between cancer type and impact of PET and PET/CT on intended management: findings of the national oncologic PET registry. J Nucl Med 2008;49:1928-1935.
Higuchi T, Nishimukai A, Ozawa H, et al. Prognostic significance of preoperative (18)F-FDG PET/CT for breast cancer subtypes. Breast 2016;30:5-12.
Ozdemir O, Batum O, Ermin S, et al. Metabolic activity of primary tumour on PET/CT has a relationship with survival in stages I-III small-cell lung carcinoma. Clin Respir J 2020; https://doi.org/10.1111/crj.13186.
Tabata K, Nishie A, Shimomura Y, et al. Prediction of pathological response to preoperative chemotherapy for pancreatic ductal adenocarcinoma using 2-[(18)F]-fluoro-2-deoxy-d-glucose positron-emission tomography. Clin Radiol 2022;77:436-442.
Evangelista L, Zucchetta P, Moletta L, et al. The role of FDG PET/CT or PET/MRI in assessing response to neoadjuvant therapy for patients with borderline or resectable pancreatic cancer: a systematic literature review. Ann Nucl Med 2021;35:767-776.
Sperti C, Friziero A, Serafini S, et al. Prognostic implications of 18-FDG positron emission tomography/computed tomography in resectable pancreatic cancer. J Clin Med 2020;9:2169.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-247.
Evans DB, Rich TA, Byrd DR, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg 1992;127:1335-1339.
Abe T, Nakata K, Kibe S, et al. Prognostic value of preoperative nutritional and immunological factors in patients with pancreatic ductal adenocarcinoma. Ann Surg Oncol 2018;25:3996-4003.
Kim TH, Han SS, Park SJ, et al. CA 19-9 level as indicator of early distant metastasis and therapeutic selection in resected pancreatic cancer. Int J Radiat Oncol Biol Phys 2011;81:e743-e748.
Groot VP, Gemenetzis G, Blair AB, et al. Defining and Predicting Early Recurrence in 957 Patients With Resected Pancreatic Ductal Adenocarcinoma. Ann Surg 2019;269:1154-1162.
Yamamoto Y, Ikoma H, Morimura R, et al. Optimal duration of the early and late recurrence of pancreatic cancer after pancreatectomy based on the difference in the prognosis. Pancreatology 2014;14:524-529.
Sugiura T, Uesaka K, Kanemoto H, et al. Serum CA19-9 is a significant predictor among preoperative parameters for early recurrence after resection of pancreatic adenocarcinoma. J Gastrointest Surg 2012;16:977-985.
Suker M, Beumer BR, Sadot E, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol 2016;17:801-810.
Ioka T, Furuse J, Fukutomi A, et al. Randomized phase II study of chemoradiotherapy with versus without induction chemotherapy for locally advanced pancreatic cancer: Japan Clinical Oncology Group trial, JCOG1106. Jpn J Clin Oncol 2021;51:235-243.
Tsuchida H, Fujii T, Mizuma M, et al. Prognostic importance of peritoneal washing cytology in patients with otherwise resectable pancreatic ductal adenocarcinoma who underwent pancreatectomy: a nationwide, cancer registry-based study from the Japan Pancreas Society. Surgery 2019;166:997-1003.
Farma JM, Santillan AA, Melis M, et al. PET/CT fusion scan enhances CT staging in patients with pancreatic neoplasms. Ann Surg Oncol 2008;15:2465-2471.
Moon D, Kim H, Han Y, et al. Preoperative carbohydrate antigen 19-9 and standard uptake value of positron emission tomography-computed tomography as prognostic markers in patients with pancreatic ductal adenocarcinoma. J Hepatobiliary Pancreat Sci 2022;29:1133-1141.
Yamamoto T, Sugiura T, Mizuno T, et al. Preoperative FDG-PET predicts early recurrence and a poor prognosis after resection of pancreatic adenocarcinoma. Ann Surg Oncol 2015;22:677-684.
Ariake K, Motoi F, Shimomura H, et al. 18-Fluorodeoxyglucose Positron Emission Tomography Predicts Recurrence in Resected Pancreatic Ductal Adenocarcinoma. J Gastrointest Surg 2018;22:279-287.
Yokose T, Kitago M, Matsusaka Y, et al. Usefulness of (18) F-fluorodeoxyglucose positron emission tomography/computed tomography for predicting the prognosis and treatment response of neoadjuvant therapy for pancreatic ductal adenocarcinoma. Cancer Med 2020;9:4059-4068.
Akita H, Takahashi H, Ohigashi H, et al. FDG-PET predicts treatment efficacy and surgical outcome of pre-operative chemoradiation therapy for resectable and borderline resectable pancreatic cancer. Eur J Surg Oncol 2017;43:1061-1067.
Kittaka H, Takahashi H, Ohigashi H, et al. Role of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in predicting the pathologic response to preoperative chemoradiation therapy in patients with resectable T3 pancreatic cancer. World J Surg 2013;37:169-178.
Panda A, Garg I, Truty MJ, et al. Borderline Resectable and Locally Advanced Pancreatic Cancer: FDG PET/MRI and CT Tumor Metrics for Assessment of Pathologic Response to Neoadjuvant Therapy and Prediction of Survival. AJR Am J Roentgenol 2021;217:730-740.
Akita H, Takahashi H, Eguchi H, et al. Difference between carbohydrate antigen 19-9 and fluorine-18 fluorodeoxyglucose positron emission tomography in evaluating the treatment efficacy of neoadjuvant treatment in patients with resectable and borderline resectable pancreatic ductal adenocarcinoma: Results of a dual-center study. Ann Gastroenterol Surg 2021;5:381-389.
Barnes CA, Aldakkak M, Clarke CN, et al. Value of pretreatment (18)F-fluorodeoxyglucose positron emission tomography in patients with localized pancreatic cancer treated with neoadjuvant therapy. Front Oncol 2020;10:500.
Wagner M, Antunes C, Pietrasz D, et al. CT evaluation after neoadjuvant FOLFIRINOX chemotherapy for borderline and locally advanced pancreatic adenocarcinoma. Eur Radiol 2017;27:3104-3116.
Isaji S, Mizuno S, Windsor JA, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 2018;18:2-11.
Hayasaki A, Isaji S, Kishiwada M, et al. Survival analysis in patients with pancreatic ductal adenocarcinoma undergoing chemoradiotherapy followed by surgery according to the international consensus on the 2017 definition of borderline resectable cancer. Cancers (Basel) 2018;10:65.
Anger F, Doring A, van Dam J, et al. Impact of borderline resectability in pancreatic head cancer on patient survival: biology matters according to the new international consensus criteria. Ann Surg Oncol 2021;28:2325-2336.
Boone BA, Steve J, Zenati MS, et al. Serum CA 19-9 response to neoadjuvant therapy is associated with outcome in pancreatic adenocarcinoma. Ann Surg Oncol 2014;21:4351-4358.
Luo G, Jin K, Deng S, et al. Roles of CA19-9 in pancreatic cancer: Biomarker, predictor and promoter. Biochim Biophys Acta Rev Cancer 2021;1875:188409.
Acknowledgements
We would like to thank Editage (www.editage.com) for the English language editing.
Author information
Authors and Affiliations
Contributions
Protocol/project development: NI.
Data collection or management: SN, TA, NI, MM, NF, NF, TI, and SB.
Statistical analysis and interpretation of data: NI, KN, SB, KI, YO, and MN.
Manuscript writing/editing: NI, KN, and MN.
Supervision: MN.
Critical revision of the article for important intellectual content: all authors.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Supplemental Figure 1
Kaplan–Meier survival curves of responders and non-responders to neoadjuvant therapy determined by the change in SUVmax of the pre- and post-treatment PET/CT. SUVmax, maximum standardized uptake value; PET/CT, positron emission tomography/computed tomography. (PNG 336 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ikenaga, N., Nakata, K., Hayashi, M. et al. Clinical Implications of FDG-PET in Pancreatic Ductal Adenocarcinoma Patients Treated with Neoadjuvant Therapy. J Gastrointest Surg 27, 337–346 (2023). https://doi.org/10.1007/s11605-023-05591-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-023-05591-2