Abstract
Background
Patients (pts) with locally advanced gastric adenocarcinoma (LAGA) often receive neoadjuvant chemotherapy. A minority of patients do not respond to chemotherapy and thus may benefit from upfront surgery. Patient-derived organoids (PDOs) are an in vitro model that may mimic the chemotherapy response of the original tumors.
Methods
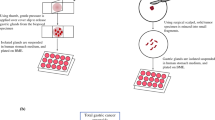
PDOs were generated from endoscopic biopsies of LAGAs prior to the initiation of chemotherapy and treated with the two chemotherapy regimens: FLOT and FOLFOX. Cell proliferation was assayed after 3–6 days. Following chemotherapy, pts underwent surgical resection, and percent pathological necrosis was determined.
Results
Successful PDOs were obtained from 13 of 24 (54%) LAGAs. Failure to generate PDOs were due to contamination (n = 3, 13%), early senescence (n = 3, 13%), and late senescence (n = 5, 21%). By H&E staining, there were significant similarities in tumor morphology and high concordance in immunohistochemical expression of 6 markers between tumors and derived PDOs. Four of 13 pts with successful PDOs did not undergo chemotherapy and surgery. For the remaining 9 pts, percent necrosis in resected tumors was ≤ 50% in 2 pts. The corresponding PDOs from these 2 pts were clearly chemoresistant outliers. The Pearson correlation coefficient between chemosensitivity of PDOs to FOLFOX (n = 2) or FLOT (n = 7) and percent tumor necrosis in resected tumors was 0.87 (p = 0.003).
Conclusions
PDOs from pts with LAGAs in many respects mimic the original tumors from which they are derived and may be used to predict resistance to neoadjuvant chemotherapy.
Synopsis
Patient-derived organoids (PDOs) can serve as personalized in vitro models of patient tumors. In this study, PDOs from locally advanced gastric cancers were able to reliably predict resistance to neoadjuvant chemotherapy.






Similar content being viewed by others
References
Sung, H., et al., Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin, 2021. 71(3): p. 209-249.
Cunningham, D., et al., Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med, 2006. 355(1): p. 11-20.
Smyth, E.C., et al., Gastric cancer. Lancet, 2020. 396(10251): p. 635-648.
Barker, N., et al., Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell, 2010. 6(1): p. 25-36.
Stange, D.E., et al., Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell, 2013. 155(2): p. 357-68.
Sato, T., et al., Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature, 2011. 469(7330): p. 415-8.
Sato, T., et al., Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature, 2009. 459(7244): p. 262-5.
van de Wetering, M., et al., Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell, 2015. 161(4): p. 933-45.
Baker, L.A., et al., Modeling pancreatic cancer with organoids. Trends Cancer, 2016. 2(4): p. 176-190.
Gao, M., et al., Development of Patient-Derived Gastric Cancer Organoids from Endoscopic Biopsies and Surgical Tissues. Ann Surg Oncol, 2018. 25(9): p. 2767-2775.
Seidlitz, T., et al., Human gastric cancer modelling using organoids. Gut, 2019. 68(2): p. 207-217.
Yan, H.H.N., et al., A Comprehensive Human Gastric Cancer Organoid Biobank Captures Tumor Subtype Heterogeneity and Enables Therapeutic Screening. Cell Stem Cell, 2018. 23(6): p. 882–897 e11.
Ganesh, K., et al., A rectal cancer organoid platform to study individual responses to chemoradiation. Nature Medicine, 2019. 25(10): p. 1607-1614.
Fujii, E., et al., A simple method for histopathological evaluation of organoids. J Toxicol Pathol, 2018. 31(1): p. 81-85.
Janjigian, Y.Y., et al., Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: a European and USA International collaborative analysis. Ann. Oncol, 2012. 23(10): p. 2656-2662.
Bang, Y.J., et al., Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet, 2010. 376(9742): p. 687-97.
Pietrantonio, F., et al., Individual Patient Data Meta-Analysis of the Value of Microsatellite Instability As a Biomarker in Gastric Cancer. J Clin Oncol, 2019. 37(35): p. 3392-3400.
Kastner, C., et al., Organoid Models for Cancer Research-From Bed to Bench Side and Back. Cancers (Basel), 2021. 13(19)
Aberle, M.R., et al., Patient-derived organoid models help define personalized management of gastrointestinal cancer. Br J Surg, 2018. 105(2): p. e48-e60.
Neal, J.T., et al., Organoid Modeling of the Tumor Immune Microenvironment. Cell, 2018. 175(7): p. 1972–1988 e16
Vlachogiannis, G., et al., Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science, 2018. 359(6378): p. 920-926.
Janjigian, Y.Y., et al., Genetic Predictors of Response to Systemic Therapy in Esophagogastric Cancer. Cancer Discov, 2018. 8(1): p. 49-58.
Schumacher, D., et al., Heterogeneous pathway activation and drug response modelled in colorectal-tumor-derived 3D cultures. PLoS Genet, 2019. 15(3): p. e1008076.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article was presented in the Plenary Session of the Society for Surgical Oncology Annual Meeting (Dallas, Texas) on March 20, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yoon, C., Lu, J., Kim, BJ. et al. Patient-Derived Organoids from Locally Advanced Gastric Adenocarcinomas Can Predict Resistance to Neoadjuvant Chemotherapy. J Gastrointest Surg 27, 666–676 (2023). https://doi.org/10.1007/s11605-022-05568-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-022-05568-7