Abstract
Background
The aim of this study was to define whether procalcitonin (PCT) is an earlier and more accurate predictor than C-reactive protein (CRP) for anastomotic leakage (AL) and major infective complications (MICs).
Methods
This was a prospective multicentric observational study conducted in three Italian centers, including all patients undergoing gastrectomy from May 2016 to April 2021. The endpoint was the assessment of the discrimination and accuracy achieved by the PCT and CRP values measured from POD1 to POD7 for predicting the occurrence of AL and MICs. Accuracy was assessed by calculating the area under the receiver operating curve (AUROC) values and Youden’s statistics. Two charts were created for risk stratification during the postoperative course.
Results
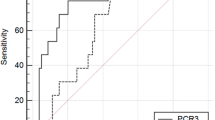
The rate of AL was 4.6%, with a median day of occurrence on POD5 (range 3–26). The overall rate of major infective complications was 19.9%, with a median day of occurrence on POD6 (range 2–30). PCT showed a significant association with AL on POD6 and POD7 and a significant association with MICs on POD2, while CRP values showed a significant association with AL on POD4 and a significant association with MICs on POD1. No difference in the prediction of AL was observed between PCT and CRP, while CRP was found to be a superior predictor of major infective complications on POD5 (p = 0.024) and POD7 (p = 0.035).
Conclusions
PCT was not superior to CRP as an early predictor of AL and major infective complications after gastrectomy. CRP should be used as the reference screening postoperative marker.


Similar content being viewed by others
References
Kamarajah SK, Navidi M, Griffin SM, Phillips AW (2020) Impact of anastomotic leak on long-term survival in patients undergoing gastrectomy for gastric cancer. Br J Surg. https://doi.org/10.1002/bjs.11749
Kim KM, An JY, Kim HI, et al (2012) Major early complications following open, laparoscopic and robotic gastrectomy. Br J Surg 99:1681–1687. https://doi.org/10.1002/BJS.8924
Oh SJ, Choi WB, Song J, et al (2009) Complications requiring reoperation after gastrectomy for gastric cancer: 17 years experience in a single institute. J GastrointestSurg 13:239–245. https://doi.org/10.1007/S11605-008-0716-3
Makuuchi R, Irino T, Tanizawa Y, et al (2019) Esophagojejunal anastomotic leakage following gastrectomy for gastric cancer. Surg Today. https://doi.org/10.1007/s00595-018-1726-8
Low DE, Allum W, De Manzoni G, et al (2019) Guidelines for Perioperative Care in Esophagectomy: Enhanced Recovery After Surgery (ERAS®) Society Recommendations. World J. Surg. https://doi.org/10.1007/s00268-018-4786-4
Mortensen K, Nilsson M, Slim K, et al (2014) Enhanced Recovery After Surgery (ERAS®) Group. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Br J Surg. https://doi.org/10.1002/bjs.9582
Gordon AC, Cross AJ, Foo EW, Roberts RH (2018) C-reactive protein is a useful negative predictor of anastomotic leak in oesophago-gastric resection. ANZ J Surg. https://doi.org/10.1111/ans.13681
Ji L, Wang T, Tian L, Gao M (2016) The early diagnostic value of C-reactive protein for anastomotic leakage post radical gastrectomy for esophagogastric junction carcinoma: A retrospective study of 97 patients. Int J Surg. https://doi.org/10.1016/j.ijsu.2016.02.021
Castelli GP, Pognani C, Meisner M, et al (2004) Procalcitonin and C-reactive protein during systemic inflammatory response syndrome, sepsis and organ dysfunction. Crit Care. https://doi.org/10.1186/cc2877
Giaccaglia V, Salvi PF, Antonelli MS, et al (2016) Procalcitonin reveals early dehiscence in colorectal surgery. the PREDICS study. Ann Surg. https://doi.org/10.1097/SLA.0000000000001365
Hoeboer SH, Groeneveld ABJ, Engels N, et al (2015) Rising C-Reactive Protein and Procalcitonin Levels Precede Early Complications After Esophagectomy. J Gastrointest Surg. https://doi.org/10.1007/s11605-015-2745-z
Yang W, Chen X, Zhang P, et al (2021) Procalcitonin as an Early Predictor of Intra-abdominal Infections Following Gastric Cancer Resection. J Surg Res. https://doi.org/10.1016/j.jss.2020.08.037
Ruiz-Tovar J, Muñoz JL, Gonzalez J, et al (2017) C-reactive protein, fibrinogen, and procalcitonin levels as early markers of staple line leak after laparoscopic sleeve gastrectomy in morbidly obese patients within an Enhanced Recovery After Surgery (ERAS) program. Surg Endosc. https://doi.org/10.1007/s00464-017-5602-1
de Mooij CM, Maassen van den Brink M, Merry A, et al (2019) Systematic Review of the Role of Biomarkers in Predicting Anastomotic Leakage Following Gastroesophageal Cancer Surgery. J Clin Med 8:2005. https://doi.org/10.3390/JCM8112005
Tu JV (1996) Advantages and disadvantages of using artificial neural networks versus logistic regression for predicting medical outcomes. J Clin Epidemiol 49:1225–1231. https://doi.org/10.1016/S0895-4356(96)00002-9
Nora D, Salluh J, Martin-Loeches I, Póvoa P (2017) Biomarker-guided antibiotic therapy—strengths and limitations. Ann Transl Med 5. https://doi.org/10.21037/ATM.2017.04.04
Li S, Rong H, Guo Q, et al (2016) Serum procalcitonin levels distinguish Gram-negative bacterial sepsis from Gram-positive bacterial and fungal sepsis. J Res Med Sci 21. https://doi.org/10.4103/1735-1995.183996
Leng Y, Chen C, Zhang Y, et al (2019) Ability of serum procalcitonin to distinguish focus of infection and pathogen types in patients with bloodstream infection. Ann Transl Med 7:135–135. https://doi.org/10.21037/ATM.2019.03.24
Rhee C (2017) Using Procalcitonin to Guide Antibiotic Therapy. Open Forum Infect Dis 4. https://doi.org/10.1093/OFID/OFW249
Cho SY, Choi JH (2014) Biomarkers of sepsis. Infect Chemother 46:1–12. https://doi.org/10.3947/IC.2014.46.1.1
Althuwaini S, Bamehriz F, Alobaid O, et al (2018) Identification of Bacterial and Fungal Pathogens in Patients with Post-Laparoscopic Sleeve Gastrectomy Leakage. Obes Surg 28:3965–3968. https://doi.org/10.1007/S11695-018-3442-2
Zappella N, Desmard M, Chochillon C, et al (2015) Positive peritoneal fluid fungal cultures in postoperative peritonitis after bariatric surgery. Clin Microbiol Infect 21:853.e1-853.e3. https://doi.org/10.1016/J.CMI.2015.05.024
Migita K, Takayama T, Matsumoto S, et al (2014) Impact of bacterial culture positivity of the drainage fluid during the early postoperative period on the development of intra-abdominal abscesses after gastrectomy. Surg Today 44:2138–2145. https://doi.org/10.1007/S00595-014-0881-9
Lee DS, Ryu JA, Chung CR, et al (2015) Risk factors for acquisition of multidrug-resistant bacteria in patients with anastomotic leakage after colorectal cancer surgery. Int J Colorectal Dis 30:497–504. https://doi.org/10.1007/S00384-015-2161-6
Kip MM, Kusters R, IJzerman MJ, Steuten LM (2015) A PCT algorithm for discontinuation of antibiotic therapy is a cost-effective way to reduce antibiotic exposure in adult intensive care patients with sepsis. J Med Econ 18:944–953. https://doi.org/10.3111/13696998.2015.1064934
Zhang K, Xi H, Wu X, et al (2016) Ability of Serum C-Reactive Protein Concentrations to Predict Complications After Laparoscopy-Assisted Gastrectomy: A Prospective Cohort Study. Medicine (Baltimore) 95. https://doi.org/10.1097/MD.0000000000003798
Silber JH, Williams SV, Krakauer H, Schwartz JS (1992) Hospital and patient characteristics associated with death after surgery. A study of adverse occurrence and failure to rescue. Med Care 30:615–627. https://doi.org/10.1097/00005650-199207000-00004
Eschborn S, Weitkamp J-H (2019) Procalcitonin versus C-reactive protein: review of kinetics and performance for diagnosis of neonatal sepsis. J Perinatol 2019 397 39:893–903. https://doi.org/10.1038/s41372-019-0363-4
D’Ugo D, Agnes A, Grieco M, et al (2020) Global updates in the treatment of gastric cancer: a systematic review. Part 2: perioperative management, multimodal therapies, new technologies, standardization of the surgical treatment and educational aspects. Updates Surg. https://doi.org/10.1007/s13304-020-00771-0
Kirkham JJ, Altman DG, Williamson PR (2010) Bias Due to Changes in Specified Outcomes during the Systematic Review Process. PLoS One 5. https://doi.org/10.1371/JOURNAL.PONE.0009810
Catalogue of Bias Collaboration, Thomas ET, Heneghan C. Outcome reporting bias. In: Catalogue Of Biases 2017. www.catalogueofbiases.org/outcomereportingbias
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Figure 1s
Graphs depicting the mean values of CRP, PCT and WBC on each postoperative day from 1 to 7 for patients with or without major infective complications. The aim of this figure is to depict the timing of the variations among the different biomarkers according to the postoperative day. (PNG 424 kb)
Table 1s
(DOCX 15 kb)
Table 2s
(DOCX 15 kb)
Table 3s
(DOCX 13 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cananzi, F.C.M., Biondi, A., Agnes, A. et al. Optimal Predictors of Postoperative Complications After Gastrectomy: Results from the Procalcitonin and C-reactive Protein for the Early Diagnosis of Anastomotic Leakage in Esophagogastric Surgery (PEDALES) Study. J Gastrointest Surg 27, 478–488 (2023). https://doi.org/10.1007/s11605-022-05547-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-022-05547-y