Abstract
Background
Changes in the size and density of esophageal malignancy during neoadjuvant chemotherapy (NCT) may be useful in predicting overall survival (OS). The aim of this study was to explore this relationship in patients with adenocarcinoma.
Methods
A retrospective single-centre cohort study was performed. Consecutive patients with esophageal adenocarcinoma who received NCT followed by en bloc resection with curative intent were identified. Pre- and post-NCT computed tomography scans were reviewed. The percentage difference between the greatest tumor diameter, esophageal wall thickness and tumor density was calculated. Multivariate Cox regression analysis identified variables independently associated with OS. A ROC analysis was performed on radiological markers to identify optimal cut-off points with Kaplan–Meier plots subsequently created.
Results
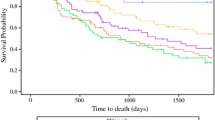
Of the 167 identified, 88 (51.5%) had disease of the gastro-esophageal junction and 149 (89.2%) were clinical T3. In total, 122 (73.1%) had node-positive disease. Increased tumor density (HR 1.01 per % change, 95% CI 1.00–1.02, p = 0.007), lymphovascular invasion (HR 3.23, 95% CI 1.34–7.52, p = 0.006) and perineural invasion (HR 2.51, 95% CI 1.03–6.08, p = 0.048) were independently associated with a decrease in OS. Patients who had a decrease in their tumor density during the time they received NCT of ≥ 20% in Hounsfield units had significantly longer OS than those who did not (75.5 months versus 34.4 months, 95% CI 38.83–105.13/18.63–35.07, p = 0.025).
Conclusions
Interval changes in the density, not size, of esophageal adenocarcinoma during the time that NCT are independently associated with OS.


Similar content being viewed by others
References
Huang R, Guo H, Chen J, Zhai T, Chen J, Lin K, et al. Intratreatment tumor volume change during definitive chemoradiotherapy is predictive for treatment outcome of patients with esophageal carcinoma. Cancer Manag Res. 2020;12: 7331–7339.
Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randominsed phase II/III trial. Lancet. 2019;393(10184):1948-1957.
Mamede M, Abreu-E-Lima P, Oliva MR, Nosé V, Mamon H, Gerbaudo VH. FDG-PET/CT tumor segmentation-derived indices of metabolic activity to assess response to neoadjuvant therapy and progression-free survival in esophageal cancer: Correlation with histopathology results. Am J Clin Oncol Cancer Clin Trials. 2007;30(4):377-88.
Beukinga RJ, Hulshoff JB, Van Dijk L V., Muijs CT, Burgerhof JGM, Kats-Ugurlu G, et al. Predicting response to neoadjuvant chemoradiotherapy in esophageal cancer with textural features derived from pretreatment 18F-FDG PET/CT imaging. J Nucl Med. 2017;58(5):723-729.
Piessen G, Petyt G, Duhamel A, Mirabel X, Huglo D, Mariette C. Ineffectiveness of 18F-fluorodeoxyglucose positron emission tomography in the evaluation of tumor response after completion of neoadjuvant chemoradiation in esophageal cancer. Ann Surg. 2013;258(1):66-76.
Botet JF, Lightdale CJ, Zauber AG, Gerdes H, Urmacher C, Brennan MF. Preoperative staging of esophageal cancer: Comparison of endoscopic US and dynamic CT. Radiology. 1991;(2):419-25.
Lin Z, Cai W, Hou W, Chen Y, Gao B, Mao R, Wang L, Li Z. CT-guided survival prediction of esophageal cancer. IEEE J Biomed Heal Inf. 2022;6(6):660–9.
Berry MF. Esophageal cancer: staging system and guidelines for staging and treatment. J Thorac Dis. 2014;(Suppl 3):S289–97.
Schwartz LH, Colville JAC, Ginsberg MS, Wang L, Mazumdar M, Kalaigian J, et al. Measuring tumor response and shape change on CT: Esophageal cancer as a paradigm. Ann Oncol. 2006;17(6):1018–23.
Iyer R, DuBrow R. Imaging of esophageal cancer. Cancer Imaging. 2004;17(6):1018-23.
Barbour AP, Jones M, Gonen M, Gotley DC, Thomas J, Thomson DB, et al. Refining esophageal cancer staging after neoadjuvant therapy: Importance of treatment response. Ann Surg Oncol. 2008;15(10):2894-902.
Taniyama Y, Murakami K, Yoshida N, Takahashi K, Matsubara H, Baba H, et al. 2021 Evaluating the effect of Neoadjuvant chemotherapy for esophageal Cancer using the RECIST system with shorter-axis measurements: a retrospective multicenter study. BMC Cancer. 21(1):1008.
Yoshida N, Taniyama Y, Murakami K, Horinouchi T, Takahashi K, Shiraishi S, et al. ASO Visual Abstract: A Novel Criterion Using Esophageal Major and Minor Axes is Useful to Evaluate the Therapeutic Effect and Prognosis after Neoadjuvant Chemotherapy Followed by Surgery in Locally Advanced Esophageal Cancer. Ann Surg Oncol. 2021;(13):8474-8482.
Kroese TE, Goense L, Van Hillegersberg R, De Keizer B, Mook S, Ruurda JP, et al. Detection of distant interval metastases after neoadjuvant therapy for esophageal cancer with 18 F-FDG PET(/CT): A systematic review and meta-analysis. Dis Esophagus. 2018;31(12):1–9.
Cerfolio RJ, Bryant AS, Ohja B, Bartolucci AA, Eloubeidi MA. The accuracy of endoscopic ultrasonography with fine-needle aspiration, integrated positron emission tomography with computed tomography, and computed tomography in restaging patients with esophageal cancer after neoadjuvant chemoradiotherapy. J Thorac Cardiovasc Surg. 2005;129(6):1232-41.
de Gouw DJJM, Klarenbeek BR, Driessen M, Bouwense SAW, van Workum F, Fütterer JJ, et al. Detecting Pathological Complete Response in Esophageal Cancer after Neoadjuvant Therapy Based on Imaging Techniques: A Diagnostic Systematic Review and Meta-Analysis. J. Thorac. Oncol. 2019; 4(7):1156-1171.
Defize IL, van Hillegersberg R, Mook S, Meijer GJ, Lin SH, Ruurda JP, et al. Restaging after chemoradiotherapy for locally advanced esophageal cancer. Ann Transl Med. 2019;7(Suppl 8):S288.
Djuric-Stefanovic A, Jankovic A, Saponjski D, Micev M, Stojanovic-Rundic S, Cosic-Micev M, et al. Analyzing the post-contrast attenuation of the esophageal wall on routine contrast-enhanced MDCT examination can improve the diagnostic accuracy in response evaluation of the squamous cell esophageal carcinoma to neoadjuvant chemoradiotherapy in comparison with esophageal wall thickness. Abdom Radiol. 2019;44(5):1722-1733.
Ferri LE, Ades S, Alcindor T, Chasen M, Marcus V, Hickeson M, et al. Perioperative docetaxel, cisplatin, and 5-fluorouracil (DCF) for locally advanced esophageal and gastric adenocarcinoma: A multicenter phase II trial. Ann Oncol. 2012;23(6):1512-7.
Spicer JD, Stiles BM, Sudarshan M, Correa AM, Ferri LE, Altorki NK, et al. Preoperative Chemoradiation Therapy Versus Chemotherapy in Patients Undergoing Modified en Bloc Esophagectomy for Locally Advanced Esophageal Adenocarcinoma: Is Radiotherapy Beneficial? Ann Thorac Surg. 2016;101(4):1262-9.
Rice TW, Gress DM, Patil DT, Hofstetter WL, Kelsen DP, Blackstone EH. Cancer of the esophagus and esophagogastric junction-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(4):304-317.
Watanabe H, Okada M, Kaji Y, Satouchi M, Sato Y, Yamabe Y, et al. New response evaluation criteria in solid tumours - Revised RECIST Guideline (version 1.1). Japanese J. Cancer Chemother. 2009;45(2):228-47.
Yanagawa M, Tatsumi M, Miyata H, Morii E, Tomiyama N, Watabe T, et al. Evaluation of response to neoadjuvant chemotherapy for esophageal cancer: PET response criteria in solid tumors versus response evaluation criteria in solid tumors. J Nucl Med. 2012;53(6):872-8.
Zhang YH, Herlin G, Rouvelas I, Nilsson M, Lundell L, Brismar TB. Texture analysis of computed tomography data using morphologic and metabolic delineation of esophageal cancer—relation to tumor type and neoadjuvant therapy response. Dis Esophagus. 2019 32(4):doy096.
Smyth EC, Lagergren J, Fitzgerald RC, Lordick F, Shah MA, Lagergren P, et al. Oesophageal cancer. Nat Rev Dis Prim. 2017;3:17048.
Barker H, Paget, J, Khan A, Harrington K. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15(7):409-425.
Thomas M, Borggreve AS, van Rossum PSN, Perneel C, Moons J, Van Daele E, et al. Radiation dose and pathological response in oesophageal cancer patients treated with neoadjuvant chemoradiotherapy followed by surgery: a multi-institutional analysis. Acta Oncol (Madr). 2019;58(10):1358-1365.
Kurokawa Y, Shibata T, Ando N, Seki S, Mukaida H, Fukuda H. Which is the optimal response criteria for evaluating preoperative treatment in esophageal cancer: RECIST or histology? Ann Surg Oncol. 2013;20(9):3009-14.
Odawara S, Kitajima K, Katsuura T, Kurahashi Y, Shinohara H, Yamakado K. Tumor response to neoadjuvant chemotherapy in patients with esophageal cancer assessed with CT and FDG-PET/CT – RECIST 1.1 vs. PERCIST 1.0. Eur J Radiol. 2018;101:65-71.
Tamandl D, Gore RM, Fueger B, Kinsperger P, Hejna M, Paireder M, et al. Change in volume parameters induced by neoadjuvant chemotherapy provide accurate prediction of overall survival after resection in patients with oesophageal cancer. Eur Radiol. 2016;26(2):311-21.
Defize IL, Boekhoff MR, Borggreve AS, van Lier ALHMW, Takahashi N, Haj Mohammad N, et al. Tumor volume regression during neoadjuvant chemoradiotherapy for esophageal cancer: a prospective study with weekly MRI. Acta Oncol (Madr). 2020;59(7):753-759.
Kim J, Bowlby R, Mungall AJ, Robertson AG, Odze RD, Cherniack AD, et al. Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541(7636):169-175.
Beer AJ, Wieder HA, Lordick F, Ott K, Fischer M, Becker K, et al. Adenocarcinomas of esophagogastric junction: Multi-detector row CT to evaluate early response to neoadjuvant chemotherapy. Radiology. 2006;(2):472-80.
Alnaji RM, Du W, Gabriel E, Singla S, Attwood K, Nava H, et al. Pathologic Complete Response Is an Independent Predictor of Improved Survival Following Neoadjuvant Chemoradiation for Esophageal Adenocarcinoma. J Gastrointest Surg. 2016;20(9):1541-6.
Sloof GW. Response monitoring of neoadjuvant therapy using CT, EUS, and FDG-PET. Best Pract Res Clin Gastroenterol. 2006;20(5):941-57.
Yip C, Davnall F, Kozarski R, Landau DB, Cook GJR, Ross P, et al. Assessment of changes in tumor heterogeneity following neoadjuvant chemotherapy in primary esophageal cancer. Dis Esophagus. 2015;28(2):172-9.
Lee S, Choi Y, Park G, Jo S, Lee SS, Park J, et al. 18F-FDG PET/CT Parameters for Predicting Prognosis in Esophageal Cancer Patients Treated With Concurrent Chemoradiotherapy. Technol Cancer Res Treat. 2021;20:15330338211024655.
Roedl JB, Colen RR, Holalkere NS, Fischman AJ, Choi NC, Blake MA. Adenocarcinomas of the esophagus: Response to chemoradiotherapy is associated with decrease of metabolic tumor volume as measured on PET-CT. Comparison to histopathologic and clinical response evaluation. Radiother Oncol. 2008;89(3):278-86.
Liu K, Li G, Fan C, Zhou C, Li J. Adapted Choi response criteria for prediction of clinical outcome in locally advanced gastric cancer patients following preoperative chemotherapy. Acta radiol. 2012; 3(2):127-34.
Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J Clin Oncol. 2007;25(13):1753-9.
Barbour AP, Walpole ET, Mai GT, Barnes EH, Watson DI, Ackland SP, et al. Preoperative cisplatin, fluorouracil, and docetaxel with or without radiotherapy after poor early response to cisplatin and fluorouracil for resectable oesophageal adenocarcinoma (AGITG DOCTOR): results from a multicentre, randomised controlled phase II trial. Ann Oncol. 2020;393(10184):1948-1957.
Lordick F, Ott K, Krause BJ, Weber WA, Becker K, Stein HJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol. 2007;393(10184):1948-1957.
Goodman KA, Ou FS, Hall NC, Bekaii-Saab T, Fruth B, Twohy E, et al. Randomized Phase II Study of PET Response-Adapted Combined Modality Therapy for Esophageal Cancer: Mature Results of the CALGB 80803 (Alliance) Trial. J Clin Oncol. 2021;39(25):2803-2815.
Funding
JT received a travel grant of $4000 from Medtronic and a regular consulting fee from Google.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the manuscript, analysis and interpretation of the results, drafting and critical revision of the manuscript and agree to be accountable for all aspects of this work.
Corresponding author
Ethics declarations
Ethical Standards
Institutional ethical approval was sought and given. The requirement for informed consent was waived in light of the anonymous and retrospective nature of the study.
Conflict of Interest
JT received a $4000 travel grant from Medtronic and receives regular consulting fees from Google. The remaining authors have no conflict of interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Meeting presentation: none.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tankel, J., Söderström, H., Reizine, E. et al. Change in Density Not Size of Esophageal Adenocarcinoma During Neoadjuvant Chemotherapy Is Associated with Improved Survival Outcomes. J Gastrointest Surg 26, 2417–2425 (2022). https://doi.org/10.1007/s11605-022-05422-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-022-05422-w