Abstract
Background
Transperineal abdominoperineal excision (TpAPE) is an emerging approach for low rectal cancers but is technically challenging. Based on an anatomical study we conducted previously, we have standardized the TpAPE procedure. Here, we aimed to validate the feasibility of the standardized TpAPE by investigating the short-term outcomes.
Methods
From January 2018 to November 2020, a total of 405 patients underwent laparoscopic or robotic rectal resection for rectal cancer in our institution. For the current study, we analyzed data for the 31 patients who underwent TpAPE. The abdominal phase was performed synchronously with the perineal phase using either a laparoscopic or robotic approach. Short-term outcomes included operative and pathological results.
Results
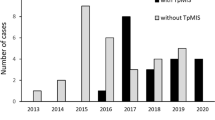
Of the 31 cases, we identified anterior quadrant tumor invasion in 21. Most of the cases were advanced, with 6 staged as cT3 and 20 as T4. Of the 27 cases not involving distant metastasis, neoadjuvant therapy was performed in 19. No inadvertent rectal perforation or urethral injury was found intraoperatively. The median procedural duration to specimen removal was 250 min (interquartile range, 204–287), and the median intraoperative blood loss was 10 ml (interquartile range, 5–40). Regarding postoperative complications, perineal wound infection developed in 11 cases. A positive circumferential resection margin was found in 3, corresponding to the positive rate of 9.7%. These three cases were among the first 12 cases involving standardized TpAPE.
Conclusions
The current results indicate that TpAPE can be performed safely and might represent a useful option for low rectal cancer resection.

Similar content being viewed by others
References
1.Yamada K, Saiki Y, Takano S et al. Long-term results of intersphincteric resection for low rectal cancer in Japan. Surg Today 2019; 49: 275-285.
2.Rullier E, Denost Q, Vendrely V et al. Low rectal cancer: classification and standardization of surgery. Dis Colon Rectum 2013; 56: 560-567.
3.Wibe A, Syse A, Andersen E et al. Oncological outcomes after total mesorectal excision for cure for cancer of the lower rectum: anterior vs. abdominoperineal resection. Dis Colon Rectum 2004; 47: 48-58.
4.Marr R, Birbeck K, Garvican J et al. The modern abdominoperineal excision: the next challenge after total mesorectal excision. Ann Surg 2005; 242: 74-82.
5.Nagtegaal ID, van de Velde CJ, Marijnen CA et al. Low rectal cancer: a call for a change of approach in abdominoperineal resection. J Clin Oncol 2005; 23: 9257-9264.
6.Shihab OC, Brown G, Daniels IR et al. Patients with low rectal cancer treated by abdominoperineal excision have worse tumors and higher involved margin rates compared with patients treated by anterior resection. Dis Colon Rectum 2010; 53: 53-56.
7.Holm T, Ljung A, Haggmark T et al. Extended abdominoperineal resection with gluteus maximus flap reconstruction of the pelvic floor for rectal cancer. Br J Surg 2007; 94: 232-238.
8.West NP, Finan PJ, Anderin C et al. Evidence of the oncologic superiority of cylindrical abdominoperineal excision for low rectal cancer. J Clin Oncol 2008; 26: 3517-3522.
9.West NP, Anderin C, Smith KJE et al. Multicentre experience with extralevator abdominoperineal excision for low rectal cancer. British Journal of Surgery 2010; 97: 588-599.
10.Buchs NC, Kraus R, Mortensen NJ et al. Endoscopically assisted extralevator abdominoperineal excision. Colorectal Dis 2015; 17: O277-280.
11.van Oostendorp SE, Roodbeen SX, Chen CC et al. Transperineal minimally invasive APE: preliminary outcomes in a multicenter cohort. Tech Coloproctol 2020; 24: 823-831.
12.Hasegawa S, Okada T, Hida K et al. Transperineal minimally invasive approach for extralevator abdominoperineal excision. Surg Endosc 2016; 30: 4620-4621.
Hamabe A, Okita K, Nishidate T et al. Transperineal minimally invasive abdominoperineal excision for rectal cancer based on anatomical analysis of the muscular structure. Asian J Endosc Surg 2021. Online ahead of print.
14.Hasegawa J, Nishimura J, Mizushima T et al. Neoadjuvant capecitabine and oxaliplatin (XELOX) combined with bevacizumab for high-risk localized rectal cancer. Cancer Chemother Pharmacol 2014; 73: 1079-1087.
15.Hata T, Takahashi H, Sakai D et al. Neoadjuvant CapeOx therapy followed by sphincter-preserving surgery for lower rectal cancer. Surg Today 2017; 47: 1372-1377.
16.Kamiya T, Uehara K, Nakayama G et al. Early results of multicenter phase II trial of perioperative oxaliplatin and capecitabine without radiotherapy for high-risk rectal cancer: CORONA I study. Eur J Surg Oncol 2016; 42: 829-835.
17.Kudo T, Takemasa I, Hata T et al. A phase I study of neoadjuvant capecitabine, oxaliplatin, and irinotecan (XELOXIRI) in patients with locally advanced rectal cancer. Oncology 2019; 97: 211-216.
18.Uehara K, Hiramatsu K, Maeda A et al. Neoadjuvant oxaliplatin and capecitabine and bevacizumab without radiotherapy for poor-risk rectal cancer: N-SOG 03 Phase II trial. Jpn J Clin Oncol 2013; 43: 964-971.
19.Schrag D, Weiser MR, Goodman KA et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol 2014; 32: 513-518.
20.Hashiguchi Y, Muro K, Saito Y et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol 2019; 25: 1-42.
21.Battersby NJ, How P, Moran B et al. Prospective validation of a low rectal cancer magnetic resonance imaging staging system and development of a local recurrence risk stratification model: the MERCURY II study. Ann Surg 2016; 263: 751-760.
22.How P, West NP, Brown G. An MRI-based assessment of standard and extralevator abdominoperineal excision specimens: time for a patient tailored approach? Ann Surg Oncol 2014; 21: 822-828.
Author information
Authors and Affiliations
Contributions
Atsushi Hamabe: project development, data collection and analysis, and manuscript writing. Kenji Okita: project development, data analysis, and manuscript editing. Toshihiko Nishidate: project development and data analysis. Koichi Okuya: project development, data collection and analysis, and manuscript editing. Emi Akizuki: project development and data analysis. Yu Sato: project development and manuscript editing. Masayuki Ishii: project development, data collection and analysis, and manuscript writing. Ryo Miura: project development and data collection. Takahiro Korai: project development and collection. Ichiro Takemasa: project development, data analysis, and manuscript editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 58186 KB)
Supplementary file2 (MOV 59530 KB)
Supplementary file3 (MP4 288009 KB)
Supplementary file4 (MOV 293865 KB)
Rights and permissions
About this article
Cite this article
Hamabe, A., Okita, K., Nishidate, T. et al. Short-Term Outcomes with Standardized Transperineal Minimally Invasive Abdominoperineal Excision for Rectal Cancer. J Gastrointest Surg 26, 713–719 (2022). https://doi.org/10.1007/s11605-021-05140-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-021-05140-9