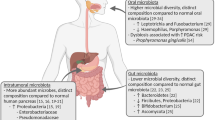
Abstract
The microbiome plays a major role in human physiology by influencing obesity, inducing inflammation, and impacting cancer therapies. During the 60th Annual Meeting of the Society of the Alimentary Tract (SSAT) at the State-of-the-Art Conference, experts in the field discussed the influence of the microbiome. This paper is a summary of the influence of the microbiome on obesity, inflammatory bowel disease, pancreatic cancer, cancer therapies, and gastrointestinal optimization. This review shows how the microbiome plays an important role in the development of diseases and surgical complications. Future studies are needed in targeting the gut microbiome to develop individualized therapies.


Similar content being viewed by others
References
Turnbaugh, P.J., et al., A core gut microbiome in obese and lean twins. Nature, 2009. 457(7228): p. 480–4.
Belkaid, Y. and T.W. Hand, Role of the microbiota in immunity and inflammation. Cell, 2014. 157(1): p. 121–141.
Viaud, S., et al., The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science, 2013. 342(6161): p. 971–6.
Stark, C.M., et al., Antibiotic and acid-suppression medications during early childhood are associated with obesity. Gut, 2019. 68(1): p. 62.
Martens, E.C., Fibre for the future. Nature, 2016. 529: p. 158.
Goodrich, J.K., et al., Human genetics shape the gut microbiome. Cell, 2014. 159(4): p. 789–99.
Rothschild, D., et al., Environment dominates over host genetics in shaping human gut microbiota. Nature, 2018. 555: p. 210.
Vidal, A.C., et al., Associations between antibiotic exposure during pregnancy, birth weight and aberrant methylation at imprinted genes among offspring. Int J Obes (Lond), 2013. 37(7): p. 907–13.
Stanislawski, M.A., et al., Gut Microbiota in the First 2 Years of Life and the Association with Body Mass Index at Age 12 in a Norwegian Birth Cohort. mBio, 2018. 9(5): p. e01751–18.
Azad, M.B., et al., Infant antibiotic exposure and the development of childhood overweight and central adiposity. Int J Obes (Lond), 2014. 38(10): p. 1290–8.
Cox, L.M. and M.J. Blaser, Antibiotics in early life and obesity. Nat Rev Endocrinol, 2015. 11(3): p. 182–90.
Thaiss, C.A., et al., Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations. Cell, 2016. 167(6): p. 1495–1510.e12.
Thaiss, Christoph A., et al., Transkingdom Control of Microbiota Diurnal Oscillations Promotes Metabolic Homeostasis. Cell, 2014. 159(3): p. 514–529.
Zarrinpar, A., et al., Diet and Feeding Pattern Affect the Diurnal Dynamics of the Gut Microbiome. Cell Metabolism, 2014. 20(6): p. 1006–1017.
Thaiss, C.A., et al., Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature, 2016. 540: p. 544.
Fragiadakis, G.K., et al., Links between environment, diet, and the hunter-gatherer microbiome. Gut Microbes, 2018: p. 1–12.
Sonnenburg, E.D., et al., Diet-induced extinctions in the gut microbiota compound over generations. Nature, 2016. 529(7585): p. 212–5.
David, L.A., et al., Diet rapidly and reproducibly alters the human gut microbiome. Nature, 2013. 505: p. 559.
Griffin, N.W., et al., Prior Dietary Practices and Connections to a Human Gut Microbial Metacommunity Alter Responses to Diet Interventions. Cell Host Microbe, 2017. 21(1): p. 84–96.
Wu, G.D., et al., Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science, 2011. 334(6052): p. 105.
Singh, R.K., et al., Influence of diet on the gut microbiome and implications for human health. Journal of Translational Medicine, 2017. 15(1): p. 73.
Caesar, R., et al., Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metabolism, 2015. 22(4): p. 658–668.
Chassaing, B., et al., Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature, 2015. 519(7541): p. 92–6.
Suez, J., et al., Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature, 2014. 514(7521): p. 181–6.
van der Beek, C.M., et al., The prebiotic inulin improves substrate metabolism and promotes short-chain fatty acid production in overweight to obese men. Metabolism, 2018. 87: p. 25–35.
Nicolucci, A.C., et al., Prebiotics Reduce Body Fat and Alter Intestinal Microbiota in Children Who Are Overweight or With Obesity. Gastroenterology, 2017. 153(3): p. 711–722.
Torres-Fuentes, C., et al., The microbiota-gut-brain axis in obesity. The Lancet Gastroenterology & Hepatology, 2017. 2(10): p. 747–756.
den Besten, G., et al., Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am J Physiol Gastrointest Liver Physiol, 2013. 305(12): p. G900–10.
Kootte, R.S., et al., Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab, 2017. 26(4): p. 611–619.e6.
Cani, P.D. and W.M. de Vos, Next-Generation Beneficial Microbes: The Case of Akkermansia muciniphila. Frontiers in Microbiology, 2017. 8(1765).
Santacruz, A., et al., Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr, 2010. 104(1): p. 83–92.
Ilhan, Z.E., et al., Distinctive microbiomes and metabolites linked with weight loss after gastric bypass, but not gastric banding. The Isme Journal, 2017. 11: p. 2047.
Bouter, K., et al., Differential metabolic effects of oral butyrate treatment in lean versus metabolic syndrome subjects. Clinical and translational gastroenterology, 2018. 9(5): p. 155–155.
Hellmann, M.D., et al., Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med, 2018. 378(22): p. 2093–2104.
Hibberd, A.A., et al., Probiotic or synbiotic alters the gut microbiota and metabolism in a randomised controlled trial of weight management in overweight adults. Beneficial Microbes, 2018. 10(2): p. 121–135.
Ferrarese R, Ceresola E.R., Preti A, Canducci F, Probiotics, prebiotics and synbiotics for weight loss and metabolic syndrome in the microbiome era. European Review for medical and Pharmacological Sciences, 2018. 22(21): p. 7588–7605.
Seifarth, C., B. Schehler, and H.J. Schneider, Effectiveness of metformin on weight loss in non-diabetic individuals with obesity. Exp Clin Endocrinol Diabetes, 2013. 121(1): p. 27–31.
Pryor, R. and F. Cabreiro, Repurposing metformin: an old drug with new tricks in its binding pockets. Biochemical Journal, 2015. 471(3): p. 307.
Zheng, J., et al., Prebiotic Mannan-Oligosaccharides Augment the Hypoglycemic Effects of Metformin in Correlation with Modulating Gut Microbiota. Journal of Agricultural and Food Chemistry, 2018. 66(23): p. 5821–5831.
Liou, A.P., et al., Conserved Shifts in the Gut Microbiota Due to Gastric Bypass Reduce Host Weight and Adiposity. Science Translational Medicine, 2013. 5(178): p. 178ra41.
Tremaroli, V., et al., Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metabolism, 2015. 22(2): p. 228–238.
Sutton, E.F., et al., Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab, 2018. 27(6): p. 1212–1221.e3.
Kaplan, L.M. and J. Brancale, Eat Well, or Get Roommates Who Do. Cell Host & Microbe, 2017. 21(2): p. 123–125.
Vrieze, A., et al., Transfer of Intestinal Microbiota From Lean Donors Increases Insulin Sensitivity in Individuals With Metabolic Syndrome. Gastroenterology, 2012. 143(4): p. 913–916.e7.
Zeevi, D., et al., Personalized Nutrition by Prediction of Glycemic Responses. Cell, 2015. 163(5): p. 1079–1094.
Russell, W., An address on a characteristic organism of cancer. Br. Med. J. , 1980. 2: p. 1356–1360
Eck, D.L., et al., Effects of immediate reconstruction on adjuvant chemotherapy in breast cancer patients. Ann Plast Surg, 2015. 74 Suppl 4: p. S201–3.
Gao, P., et al., Impact of timing of adjuvant chemotherapy on survival in stage III colon cancer: a population-based study. BMC Cancer, 2018. 18(1): p. 234.
Arhi, C.S., et al., Complications after discharge and delays in adjuvant chemotherapy following colonic resection: a cohort study of linked primary and secondary care data. Colorectal Dis, 2019. 21(3): p. 307–314.
Ortega, G., et al., An evaluation of surgical site infections by wound classification system using the ACS-NSQIP. J Surg Res, 2012. 174(1): p. 33–8.
Krezalek, M.A., et al., Can Methicillin-resistant Staphylococcus aureus Silently Travel From the Gut to the Wound and Cause Postoperative Infection? Modeling the "Trojan Horse Hypothesis". Ann Surg, 2018. 267(4): p. 749–758.
Roy, S., et al., Staphylococcus aureus Biofilm Infection Compromises Wound Healing by Causing Deficiencies in Granulation Tissue Collagen. Ann Surg, 2019.
Cancer Facts & Figures 2019. American Cancer Society. 2019.
Shogan, B.D., et al., Collagen degradation and MMP9 activation by Enterococcus faecalis contribute to intestinal anastomotic leak. Sci Transl Med, 2015. 7(286): p. 286ra68.
Van Cutsem, E., et al., Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med, 2009. 360(14): p. 1408–17.
Prahallad, A., et al., Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature, 2012. 483(7387): p. 100–3.
Wallace, B.D., et al., Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science, 2010. 330(6005): p. 831–5.
Gui, Q.F., et al., Well-balanced commensal microbiota contributes to anti-cancer response in a lung cancer mouse model. Genet Mol Res, 2015. 14(2): p. 5642–51.
Geller, L.T., et al., Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science, 2017. 357(6356): p. 1156–1160.
Stockis, J., R. Roychoudhuri, and T.Y.F. Halim, Regulation of regulatory T cells in cancer. Immunology, 2019.
Postow, M.A., et al., Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med, 2015. 372(21): p. 2006–17.
Motzer, R.J., et al., Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med, 2018. 378(14): p. 1277–1290.
Forde, P.M., et al., Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med, 2018. 378(21): p. 1976–1986.
Rescigno, M., Intestinal microbiota and its effects on the immune system. Cell Microbiol, 2014. 16(7): p. 1004–13.
Tomkovich, S. and C. Jobin, Microbiota and host immune responses: a love-hate relationship. Immunology, 2016. 147(1): 1–10.
Vetizou, M., et al., Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science, 2015. 350(6264): p. 1079–84.
Sivan, A., et al., Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science, 2015. 350(6264): p. 1084–9.
Gopalakrishnan, V., et al., Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science, 2018. 359(6371): p. 97–103.
Flint, H.J., et al., The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol, 2012. 9(10): p. 577–89.
Claesson, M.J., et al., Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A, 2011. 108 Suppl 1: p. 4586–91.
Loftus, E.V., Jr., Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology, 2004. 126(6): p. 1504–17.
Jostins, L., et al., Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature, 2012. 491(7422): p. 119–24.
Khor, B., A. Gardet, and R.J. Xavier, Genetics and pathogenesis of inflammatory bowel disease. Nature, 2011. 474(7351): p. 307–17.
Halme, L., et al., Family and twin studies in inflammatory bowel disease. World J Gastroenterol, 2006. 12(23): p. 3668–72.
Sartor, R.B., Microbial influences in inflammatory bowel diseases. Gastroenterology, 2008. 134(2): p. 577–94.
Ogura, Y., et al., A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature, 2001. 411(6837): p. 603–6.
Strober, W., I. Fuss, and P. Mannon, The fundamental basis of inflammatory bowel disease. J Clin Invest, 2007. 117(3): p. 514–21.
Sartor, R.B., Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology, 2004. 126(6): p. 1620–33.
Kang, S., et al., Dysbiosis of fecal microbiota in Crohn's disease patients as revealed by a custom phylogenetic microarray. Inflamm Bowel Dis, 2010. 16(12): p. 2034–42.
Martinez, C., et al., Unstable composition of the fecal microbiota in ulcerative colitis during clinical remission. Am J Gastroenterol, 2008. 103(3): p. 643–8.
Sepehri, S., et al., Microbial diversity of inflamed and noninflamed gut biopsy tissues in inflammatory bowel disease. Inflamm Bowel Dis, 2007. 13(6): p. 675–83.
Kitajima, S., et al., Dextran sodium sulfate-induced colitis in germ-free IQI/Jic mice. Exp Anim, 2001. 50(5): p. 387–95.
Cadwell, K., et al., Virus-plus-susceptibility gene interaction determines Crohn's disease gene Atg16L1 phenotypes in intestine. Cell, 2010. 141(7): p. 1135–45.
Trojanowska, D., et al., The role of Candida in inflammatory bowel disease. Estimation of transmission of C. albicans fungi in gastrointestinal tract based on genetic affinity between strains. Med Sci Monit, 2010. 16(10): p. CR451–7.
Darfeuille-Michaud, A., et al., High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology, 2004. 127(2): p. 412–21.
Human Microbiome Project, C., Structure, function and diversity of the healthy human microbiome. Nature, 2012. 486(7402): p. 207–14.
Morgan, X.C., et al., Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol, 2012. 13(9): p. R79.
Whelan, K. and E.M. Quigley, Probiotics in the management of irritable bowel syndrome and inflammatory bowel disease. Curr Opin Gastroenterol, 2013. 29(2): p. 184–9.
van Nood, E., M.G. Dijkgraaf, and J.J. Keller, Duodenal infusion of feces for recurrent Clostridium difficile. N Engl J Med, 2013. 368(22): p. 2145.
Kump, P.K., et al., Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis, 2013. 19(10): p. 2155–65.
Michaud, D.S., Epidemiology of pancreatic cancer. Minerva Chir, 2004. 59(2): p. 99–111.
Feng, M., et al., PD-1/PD-L1 and immunotherapy for pancreatic cancer. Cancer Lett, 2017. 407: p. 57–65.
Guo, S., et al., Immunotherapy in pancreatic cancer: Unleash its potential through novel combinations. World J Clin Oncol, 2017. 8(3): p. 230–240.
Nesselhut, J., et al., Systemic treatment with anti-PD-1 antibody nivolumab in combination with vaccine therapy in advanced pancreatic cancer. Journal of Clinical Oncology, 2016. 34(15_suppl): p. 3092–3092.
Pushalkar, S., et al., The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov, 2018.
Tanoue, T., et al., A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature, 2019. 565(7741): p. 600–605.
Hibberd, A.A., et al., Intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. BMJ Open Gastroenterol, 2017. 4(1): p. e000145.
Hu, Y.J., et al., [Early outcomes of elective surgery for colon cancer with preoperative mechanical bowel preparation: a randomized clinical trial]. Nan Fang Yi Ke Da Xue Xue Bao, 2017. 37(1): p. 13–17.
Guenaga, K.F., D. Matos, and P. Wille-Jorgensen, Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev, 2011(9): p. CD001544.
Rollins, K.E., H. Javanmard-Emamghissi, and D.N. Lobo, Impact of mechanical bowel preparation in elective colorectal surgery: A meta-analysis. World J Gastroenterol, 2018. 24(4): p. 519–536.
Nichols, R.L., et al., Effect of preoperative neomycin-erythromycin intestinal preparation on the incidence of infectious complications following colon surgery. 1973. Surg Infect (Larchmt), 2000. 1(2): p. 133–41; discussion 143, 145–6, 147–8.
Anjum, N., et al., A Randomized Control Trial of Preoperative Oral Antibiotics as Adjunct Therapy to Systemic Antibiotics for Preventing Surgical Site Infection in Clean Contaminated, Contaminated, and Dirty Type of Colorectal Surgeries. Dis Colon Rectum, 2017. 60(12): p. 1291–1298.
Kiran, R.P., et al., Combined preoperative mechanical bowel preparation with oral antibiotics significantly reduces surgical site infection, anastomotic leak, and ileus after colorectal surgery. Ann Surg, 2015. 262(3): p. 416–25; discussion 423-5.
Ikeda, A., et al., Randomized clinical trial of oral and intravenous versus intravenous antibiotic prophylaxis for laparoscopic colorectal resection. Br J Surg, 2016. 103(12): p. 1608–1615.
Cannon, J.A., et al., Preoperative oral antibiotics reduce surgical site infection following elective colorectal resections. Dis Colon Rectum, 2012. 55(11): p. 1160–6.
Garfinkle, R., et al., Is There a Role for Oral Antibiotic Preparation Alone Before Colorectal Surgery? ACS-NSQIP Analysis by Coarsened Exact Matching. Dis Colon Rectum, 2017. 60(7): p. 729–737.
Mahajna, A., et al., Bowel preparation is associated with spillage of bowel contents in colorectal surgery. Dis Colon Rectum, 2005. 48(8): p. 1626–31.
Shogan, B.D., et al., Do we really know why colorectal anastomoses leak? J Gastrointest Surg, 2013. 17(9): p. 1698–707.
Jalanka, J., et al., Effects of bowel cleansing on the intestinal microbiota. Gut, 2015. 64(10): p. 1562–8.
Drago, L., et al., Persisting changes of intestinal microbiota after bowel lavage and colonoscopy. Eur J Gastroenterol Hepatol, 2016. 28(5): p. 532–7.
Sinsimer, D., et al., The common prophylactic therapy for bowel surgery is ineffective for clearing Bacteroidetes, the primary inducers of systemic inflammation, and causes faster death in response to intestinal barrier damage in mice. BMJ Open Gastroenterol, 2014. 1(1): p. e000009.
Shobar, R.M., et al., The Effects of Bowel Preparation on Microbiota-Related Metrics Differ in Health and in Inflammatory Bowel Disease and for the Mucosal and Luminal Microbiota Compartments. Clin Transl Gastroenterol, 2016. 7: p. e143.
Olivas, A.D., et al., Intestinal tissues induce an SNP mutation in Pseudomonas aeruginosa that enhances its virulence: possible role in anastomotic leak. PLoS One, 2012. 7(8): p. e44326.
van Praagh, J.B., et al., Mucus Microbiome of Anastomotic Tissue During Surgery Has Predictive Value for Colorectal Anastomotic Leakage. Ann Surg, 2019. 269(5): p. 911–916.
Pohl, J.M., et al., Irf4-dependent CD103(+)CD11b(+) dendritic cells and the intestinal microbiome regulate monocyte and macrophage activation and intestinal peristalsis in postoperative ileus. Gut, 2017. 66(12): p. 2110–2120.
Okada, M., et al., Experimental study of the influence of intestinal flora on the healing of intestinal anastomoses. Br J Surg, 1999. 86(7): p. 961–5.
Wren, S.M., et al., Preoperative oral antibiotics in colorectal surgery increase the rate of Clostridium difficile colitis. Arch Surg, 2005. 140(8): p. 752–6.
Kotzampassi, K., et al., A Four-Probiotics Regimen Reduces Postoperative Complications After Colorectal Surgery: A Randomized, Double-Blind, Placebo-Controlled Study. World J Surg, 2015. 39(11): p. 2776–83.
de Andrade Calaca, P.R., et al., Probiotics as a preventive strategy for surgical infection in colorectal cancer patients: a systematic review and meta-analysis of randomized trials. Transl Gastroenterol Hepatol, 2017. 2: p. 67.
Suez, J., et al., Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT. Cell, 2018. 174(6): p. 1406–1423 e16.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Miller, M.O., Kashyap, P.C., Becker, S.L. et al. SSAT State-of-the-Art Conference: Advancements in the Microbiome. J Gastrointest Surg 25, 1885–1895 (2021). https://doi.org/10.1007/s11605-020-04551-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-020-04551-4