Abstract
Introduction
Severe burns lead to marked impairment of gastrointestinal motility, such as delayed gastric emptying and small and large intestinal ileus. However, the cellular mechanism of these pathologic changes remains largely unknown.
Methods
Male Sprague Dawley rats approximately 3 months old and weighing 300–350 g were randomized to either a 60% total body surface area full-thickness scald burn or sham procedure and were sacrificed 24 h after the procedure. Gastric emptying, gastric antrum contractility ileal smooth muscle contractility, and colonic contractility were measured. Muscularis externa was isolated from the ileal segment to prepare smooth muscle protein extracts for Western blot analysis.
Results
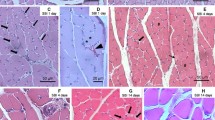
Compared with sham controls, the baseline rhythmic contractile activities of the antral, ileal, and colonic smooth muscle strips were impaired in the burned rats. Simultaneously, our data showed that ileal muscularis ECM proteins fibronectin and laminin were significantly up-regulated in burned rats compared with sham rats. TGF-β signaling is an important stimulating factor for ECM protein expression. Our results revealed that TGF-β signaling was activated in the ileal muscle of burned rats evidenced by the activation of Smad2/3 expression and phosphorylation. In addition, the total and phosphorylated AKT, which is an important downstream factor of ECM signaling in smooth muscle cells, was also up-regulated in burned rats’ ileal muscle. Notably, these changes were not seen in the colonic or gastric tissues.
Conclusion
Deposition of fibrosis-related proteins after severe burn is contributors to decreased small intestinal motility.




Similar content being viewed by others
References
Alican I, Coskun T, Yegen C, Aktan AO, Yalin R, Yegen BC. The effect of thermal injury on gastric emptying in rats. Burns 1995;21(3):171–4.
Czaja AJ, McAlhany JC, Pruitt BA, Jr. Acute gastroduodenal disease after thermal injury. An endoscopic evaluation of incidence and natural history. N Engl J Med 1974;291(18):925–9. doi:https://doi.org/10.1056/NEJM197410312911801.
Chen CF, Chapman BJ, Munday KA, Fang HS. The effects of thermal injury on gastrointestinal motor activity in the rat. Burns Incl Therm Inj 1982;9(2):142–6.
Unluer EE, Alican I, Yegen C, Yegen BC. The delays in intestinal motility and neutrophil infiltration following burn injury in rats involve endogenous endothelins. Burns 2000;26(4):335–40.
Wolf SE, Ikeda H, Matin S, Debroy MA, Rajaraman S, Herndon DN et al. Cutaneous burn increases apoptosis in the gut epithelium of mice. J Am Coll Surg 1999;188(1):10–6.
Huang HH, Lee YC, Chen CY. Effects of burns on gut motor and mucosa functions. Neuropeptides 2018;72:47–57. doi:https://doi.org/10.1016/j.npep.2018.09.004.
Oliveira HM, Sallam HS, Espana-Tenorio J, Chinkes D, Chung DH, Chen JD et al. Gastric and small bowel ileus after severe burn in rats: the effect of cyclooxygenase-2 inhibitors. Burns 2009;35(8):1180–4. doi:https://doi.org/10.1016/j.burns.2009.02.022.
Sallam HS, Oliveira HM, Liu S, Chen JD. Mechanisms of burn-induced impairment in gastric slow waves and emptying in rats. Am J Physiol Regul Integr Comp Physiol 2010;299(1):R298–305. doi:https://doi.org/10.1152/ajpregu.00135.2010.
Sahu K, Kaurav M, Pandey RS. Protease loaded permeation enhancer liposomes for treatment of skin fibrosis arisen from second degree burn. Biomed Pharmacother 2017;94:747–57. doi:https://doi.org/10.1016/j.biopha.2017.07.141.
Sokhn S, Nasseh I. Dermal fibrosis and calcification secondary to burn injury. Quintessence Int 2009;40(6):503–6.
Gabriel VA. Transforming growth factor-beta and angiotensin in fibrosis and burn injuries. J Burn Care Res 2009;30(3):471–81. doi:https://doi.org/10.1097/BCR.0b013e3181a28ddb.
Ulrich D, Noah EM, von Heimburg D, Pallua N. TIMP-1, MMP-2, MMP-9, and PIIINP as serum markers for skin fibrosis in patients following severe burn trauma. Plast Reconstr Surg 2003;111(4):1423–31. doi:https://doi.org/10.1097/01.PRS.0000049450.95669.07.
Duke JM, Randall SM, Fear MW, Boyd JH, Rea S, Wood FM. Long-term Effects of Pediatric Burns on the Circulatory System. Pediatrics 2015;136(5):e1323–30. doi:https://doi.org/10.1542/peds.2015-1945.
Duke JM, Randall SM, Fear MW, Boyd JH, Rea S, Wood FM. Understanding the long-term impacts of burn on the cardiovascular system. Burns 2016;42(2):366–74. doi:https://doi.org/10.1016/j.burns.2015.08.020.
Hundeshagen G, Herndon DN, Clayton RP, Wurzer P, McQuitty A, Jennings K et al. Long-term effect of critical illness after severe paediatric burn injury on cardiac function in adolescent survivors: an observational study. Lancet Child Adolesc Health 2017;1(4):293–301. doi:https://doi.org/10.1016/S2352-4642(17)30122-0.
Jeschke MG, Micak RP, Finnerty CC, Herndon DN. Changes in liver function and size after a severe thermal injury. Shock 2007;28(2):172–7. doi:https://doi.org/10.1097/shk.0b013e318047b9e2.
Price LA, Thombs B, Chen CL, Milner SM. Liver disease in burn injury: evidence from a national sample of 31,338 adult patients. J Burns Wounds 2007;7:e1.
Peyton SR, Kim PD, Ghajar CM, Seliktar D, Putnam AJ. The effects of matrix stiffness and RhoA on the phenotypic plasticity of smooth muscle cells in a 3-D biosynthetic hydrogel system. Biomaterials 2008;29(17):2597–607. doi:https://doi.org/10.1016/j.biomaterials.2008.02.005.
Peyton SR, Putnam AJ. Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. J Cell Physiol 2005;204(1):198–209. doi:https://doi.org/10.1002/jcp.20274.
Herrick WG, Rattan S, Nguyen TV, Grunwald MS, Barney CW, Crosby AJ et al. Smooth Muscle Stiffness Sensitivity is Driven by Soluble and Insoluble ECM Chemistry. Cell Mol Bioeng 2015;8(3):333–48. doi:https://doi.org/10.1007/s12195-015-0397-4.
Gaudet C, Marganski WA, Kim S, Brown CT, Gunderia V, Dembo M et al. Influence of type I collagen surface density on fibroblast spreading, motility, and contractility. Biophys J 2003;85(5):3329–35. doi:https://doi.org/10.1016/S0006-3495(03)74752-3.
Scheibe K, Kersten C, Schmied A, Vieth M, Primbs T, Carle B et al. Inhibiting Interleukin 36 Receptor Signaling Reduces Fibrosis in Mice With Chronic Intestinal Inflammation. Gastroenterology 2019;156(4):1082–97 e11. doi:https://doi.org/10.1053/j.gastro.2018.11.029.
Rieder F, Fiocchi C, Rogler G. Mechanisms, Management, and Treatment of Fibrosis in Patients With Inflammatory Bowel Diseases. Gastroenterology 2017;152(2):340–50 e6. doi:https://doi.org/10.1053/j.gastro.2016.09.047.
Mascarenhas DD, Elayadi A, Singh BK, Prasai A, Hegde SD, Herndon DN et al. Nephrilin peptide modulates a neuroimmune stress response in rodent models of burn trauma and sepsis. Int J Burns Trauma 2013;3(4):190–200.
Bohanon FJ, Nunez Lopez O, Herndon DN, Wang X, Bhattarai N, Ayadi AE et al. Burn Trauma Acutely Increases the Respiratory Capacity and Function of Liver Mitochondria. Shock 2018;49(4):466–73. doi:https://doi.org/10.1097/SHK.0000000000000935.
Shi XZ, Lin YM, Powell DW, Sarna SK. Pathophysiology of motility dysfunction in bowel obstruction: role of stretch-induced COX-2. Am J Physiol Gastrointest Liver Physiol 2011;300(1):G99-G108. doi:https://doi.org/10.1152/ajpgi.00379.2010.
Lin YM, Fu Y, Winston J, Radhakrishnan R, Sarna SK, Huang LM et al. Pathogenesis of abdominal pain in bowel obstruction: role of mechanical stress-induced upregulation of nerve growth factor in gut smooth muscle cells. Pain 2017;158(4):583–92. doi:https://doi.org/10.1097/j.pain.0000000000000797.
Hegde S, Lin YM, Golovko G, Khanipov K, Cong Y, Savidge T et al. Microbiota dysbiosis and its pathophysiological significance in bowel obstruction. Sci Rep 2018;8(1):13044. doi:https://doi.org/10.1038/s41598-018-31033-0.
Li H, Yin J, Zhang Z, Winston JH, Shi XZ, Chen JD. Auricular vagal nerve stimulation ameliorates burn-induced gastric dysmotility via sympathetic-COX-2 pathways in rats. Neurogastroenterol Motil 2016;28(1):36–42. doi:https://doi.org/10.1111/nmo.12693.
Cummins CB, Wang X, Nunez Lopez O, Graham G, Tie HY, Zhou J et al. Luteolin-Mediated Inhibition of Hepatic Stellate Cell Activation via Suppression of the STAT3 Pathway. Int J Mol Sci 2018;19(6). doi:https://doi.org/10.3390/ijms19061567.
Cummins CB, Wang X, Sommerhalder C, Bohanon FJ, Nunez Lopez O, Tie HY et al. Natural Compound Oridonin Inhibits Endotoxin-Induced Inflammatory Response of Activated Hepatic Stellate Cells. Biomed Res Int 2018;2018:6137420. doi:https://doi.org/10.1155/2018/6137420.
Cummins CB, Wang X, Xu J, Hughes BD, Ding Y, Chen H et al. Antifibrosis Effect of Novel Oridonin Analog CYD0618 Via Suppression of the NF-kappaB Pathway. J Surg Res 2018;232:283–92. doi:https://doi.org/10.1016/j.jss.2018.06.040.
Sallam HS, Kramer GC, Chen JD. Gastric emptying and intestinal transit of various enteral feedings following severe burn injury. Dig Dis Sci 2011;56(11):3172–8. doi:https://doi.org/10.1007/s10620-011-1755-2.
Sallam HS, Oliveira HM, Gan HT, Herndon DN, Chen JD. Ghrelin improves burn-induced delayed gastrointestinal transit in rats. Am J Physiol Regul Integr Comp Physiol 2007;292(1):R253–7. doi:https://doi.org/10.1152/ajpregu.00100.2006.
Gan HT, Chen JD. Roles of nitric oxide and prostaglandins in pathogenesis of delayed colonic transit after burn injury in rats. Am J Physiol Regul Integr Comp Physiol 2005;288(5):R1316–24. doi:https://doi.org/10.1152/ajpregu.00733.2004.
Oktar BK, Cakir B, Mutlu N, Celikel C, Alican I. Protective role of cyclooxygenase (COX) inhibitors in burn-induced intestinal and liver damage. Burns 2002;28(3):209–14.
Duband JL, Gimona M, Scatena M, Sartore S, Small JV. Calponin and SM 22 as differentiation markers of smooth muscle: spatiotemporal distribution during avian embryonic development. Differentiation 1993;55(1):1–11.
Rokolya A, Ahn HY, Moreland S, van Breemen C, Moreland RS. A hypothesis for the mechanism of receptor and G-protein-dependent enhancement of vascular smooth muscle myofilament Ca2+ sensitivity. Can J Physiol Pharmacol 1994;72(11):1420–6.
Shimomura E, Shiraishi M, Iwanaga T, Seto M, Sasaki Y, Ikeda M et al. Inhibition of protein kinase C-mediated contraction by Rho kinase inhibitor fasudil in rabbit aorta. Naunyn Schmiedebergs Arch Pharmacol 2004;370(5):414–22. doi:https://doi.org/10.1007/s00210-004-0975-9.
Sohn UD, Cao W, Tang DC, Stull JT, Haeberle JR, Wang CL et al. Myosin light chain kinase- and PKC-dependent contraction of LES and esophageal smooth muscle. Am J Physiol Gastrointest Liver Physiol 2001;281(2):G467–78. doi:https://doi.org/10.1152/ajpgi.2001.281.2.G467.
Ringvold HC, Khalil RA. Protein Kinase C as Regulator of Vascular Smooth Muscle Function and Potential Target in Vascular Disorders. Adv Pharmacol 2017;78:203–301. doi:https://doi.org/10.1016/bs.apha.2016.06.002.
Uray KS, Laine GA, Xue H, Allen SJ, Cox CS, Jr. Intestinal edema decreases intestinal contractile activity via decreased myosin light chain phosphorylation. Crit Care Med 2006;34(10):2630–7. doi:https://doi.org/10.1097/01.CCM.0000239195.06781.8C.
Touyz RM, Alves-Lopes R, Rios FJ, Camargo LL, Anagnostopoulou A, Arner A et al. Vascular smooth muscle contraction in hypertension. Cardiovasc Res 2018;114(4):529–39. doi:https://doi.org/10.1093/cvr/cvy023.
Hruz P, Dann SM, Eckmann L. STAT3 and its activators in intestinal defense and mucosal homeostasis. Curr Opin Gastroenterol 2010;26(2):109–15. doi:https://doi.org/10.1097/MOG.0b013e3283365279.
Xiao K, Song ZH, Jiao LF, Ke YL, Hu CH. Developmental changes of TGF-beta1 and Smads signaling pathway in intestinal adaption of weaned pigs. PLoS One 2014;9(8):e104589. doi:https://doi.org/10.1371/journal.pone.0104589.
Tinoco-Veras CM, Santos A, Stipursky J, Meloni M, Araujo APB, Foschetti DA et al. Transforming Growth Factor beta1/SMAD Signaling Pathway Activation Protects the Intestinal Epithelium from Clostridium difficile Toxin A-Induced Damage. Infect Immun 2017;85(10). doi:https://doi.org/10.1128/IAI.00430-17.
Mann ER, Bernardo D, English NR, Landy J, Al-Hassi HO, Peake ST et al. Compartment-specific immunity in the human gut: properties and functions of dendritic cells in the colon versus the ileum. Gut 2016;65(2):256–70. doi:https://doi.org/10.1136/gutjnl-2014-307916.
Santaolalla R, Fukata M, Abreu MT. Innate immunity in the small intestine. Curr Opin Gastroenterol 2011;27(2):125–31. doi:https://doi.org/10.1097/MOG.0b013e3283438dea.
Farina JA, Jr., Rosique MJ, Rosique RG. Curbing inflammation in burn patients. Int J Inflam 2013;2013:715645. doi:https://doi.org/10.1155/2013/715645.
Latella G, Rieder F. Intestinal fibrosis: ready to be reversed. Curr Opin Gastroenterol 2017;33(4):239–45. doi:https://doi.org/10.1097/MOG.0000000000000363.
Wang Z, Li R, Zhong R. Extracellular matrix promotes proliferation, migration and adhesion of airway smooth muscle cells in a rat model of chronic obstructive pulmonary disease via upregulation of the PI3K/AKT signaling pathway. Mol Med Rep 2018;18(3):3143–52. doi:https://doi.org/10.3892/mmr.2018.9320.
Wen JJ, Radhakrishnan GL, Cummins CB, Radhakrishnan RS. Sildenafil Prevents Adverse Cardiac Remodeling and LV Dysfunction in an In Vivo Model of Burn Injury. J Burn Care Res 2019;40(S1):S27.
Author information
Authors and Affiliations
Contributions
CBC, YG, XW, YL, XS, and RSR conceived of and designed this work. CBC, XW, YL, and XS conducted the experiments. CBC drafted the manuscript. CBC, YG, XW, YL, XS, and RSR critically reviewed the manuscript, provided approval of the final version, and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
All animal research procedures adhered to the National Institutes of Health guidelines for experimental animal use and were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch at Galveston, TX (Protocol # 1509059).
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper was presented as a plenary presentation through the Surgical Society for the Alimentary Tract at Digestive Disease Week on May 21, 2019.
Rights and permissions
About this article
Cite this article
Cummins, C.B., Gu, Y., Wang, X. et al. Burn-Induced Impairment of Ileal Muscle Contractility Is Associated with Increased Extracellular Matrix Components. J Gastrointest Surg 24, 188–197 (2020). https://doi.org/10.1007/s11605-019-04400-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-019-04400-z