Abstract
Background
Vascular reconstruction during pancreaticoduodenectomy is increasingly utilized to improve pancreatic cancer resectability. However, few multi-institutional studies have evaluated the morbidity and mortality of arterial and venous reconstruction during this procedure.
Methods
A retrospective analysis was performed utilizing the targeted pancreas module of the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) for pancreaticoduodenectomy from 2014 to 2015. Demographics, comorbidities, and 30-day outcomes for patients who underwent venous or arterial reconstruction and both were compared to no reconstruction.
Results
A total of 3002 patients were included in our study: 384 with venous reconstruction, 52 with arterial, 81 with both, and 2566 without. Compared to patients without reconstruction, those who underwent venous reconstruction had more congestive heart failure (1.8% vs 0.2%, P < 0.01), those with arterial reconstruction had higher rates of pulmonary disease (11.5% vs. 4.5%, P = 0.02), and neoadjuvant chemotherapy was more common in both venous (34% vs 12%, P < 0.01) and arterial reconstruction (21% vs 12%, P = 0.04). In multivariable analysis, there was no increase in morbidity or mortality following venous reconstruction. However, arterial reconstruction was associated with increased 30-day mortality with an odds ratio (OR): 6.7, 95%; confidence interval (CI): 1.8–25. Morbidity was increased as represented with return to the operating room (OR: 4.5, 95%; CI: 1.5–15), pancreatic fistula (OR: 4.4, 95%; CI: 1.7–11), and reintubation (OR: 3.9, 95%; CI: 1.1–14).
Conclusions
Venous reconstruction during pancreaticoduodenectomy does not increase perioperative morbidity or mortality and should be considered for patients previously considered to be unresectable or those where R0 resection would otherwise not be possible due to venous involvement. Careful consideration should be made prior to arterial reconstruction given the significant increase in perioperative complications and death within 30 days.
Similar content being viewed by others
Introduction
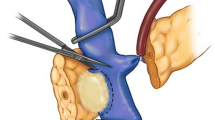
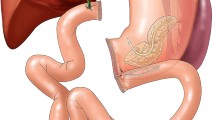
Pancreatic tumors with invasion of major vascular structures including the portal vein, superior mesenteric vein, or superior mesenteric artery have historically been considered unresectable due to the inability to achieve negative margins. However, recent practice has suggested that resection with microscopically negative margins (R0) can be successfully achieved with vascular reconstruction at the time of the index pancreaticoduodenectomy.1,2,3 Resection of these tumors is currently performed at selected centers around the country; however, this practice remains controversial due to the complex nature of the operation, the degree of local tumor invasion, and more advanced stage disease.1, 4 Moreover, the impact of vascular reconstruction on long-term survival and potential morbidity and mortality of the procedure is widely debated.4, 5 To account for the evolution of surgical technique and increasing use of vascular reconstruction, the National Comprehensive Cancer Network (NCCN) and International Study Group of Pancreatic Surgery currently consider tumors involving major vascular structures to be “borderline resectable”. Moreover, each recommend reconstruction when possible; however, they note limitations in current data as well as a lack of universal acceptance of these techniques.6, 7
The effects of vascular reconstruction on short-term outcomes following pancreaticoduodenectomy remain unclear with multiple studies reporting conflicting results.8,9,10 Additionally, much of the published literature is composed of single-center studies that have been limited by small sample size and the ability to generate risk-adjusted outcomes that incorporate both demographic and comorbidity patient data with operative findings.
Given the disparity in the effects of vascular reconstruction on post-operative outcomes and the limitations of prior studies, we conducted a retrospective analysis of a large, multi-institutional national database, which allowed adjustment for anatomic and operative details. Our primary aim was to identify whether venous and arterial reconstruction following pancreaticoduodenectomy is associated with worse 30-day morbidity and mortality.
Materials and Methods
Study Population
All patients undergoing pancreaticoduodenectomy from 2014 to 2015 were identified in the targeted pancreas module of the American College of Surgeons National Surgical Quality Improvement Program (NSQIP). The targeted NSQIP database is a national clinical registry developed in 2011, which collects patient demographics, operative details, and 30-day outcomes from patients undergoing surgical procedures at more than 65 self-selected hospitals. Further information is available at www.facs.org/quality-programs/acs-nsqip. Patients with missing data on vascular reconstruction were excluded (n = 90). Venous reconstruction was defined as reconstruction of the portal vein or superior mesenteric vein. Arterial reconstruction was defined as reconstruction of the superior mesenteric, celiac, or hepatic artery. Simultaneous reconstructions of both artery and vein were also included (n = 81). Any reconstruction or injury requiring only suture repair was not considered a vascular reconstruction.
Variables
Demographic data and comorbid conditions were compared between those patients who underwent vascular reconstruction and those who did not. Smoking was defined by current ongoing tobacco use. Glomerular filtration rate (GFR—mL/min per 1.73 m2) was calculated according to the Modification of Diet in Renal Disease equation.11 The presence of chronic kidney disease was identified according to the kidney disease: improving global outcomes (KDIGO) and acute kidney injury network (AKIN) clinical practice guidelines.12,13,14
Operative variables were also compared. The final histology was defined as either malignant or benign pathology. If both benign and malignant disease were noted, the pathology was classified as malignancy. Pre-operative chemotherapy and radiation were documented if they were completed for pancreatic cancer in the 90 days prior to operation. Pre-operative obstructive jaundice was defined by a bilirubin greater than 2 mg/dL in the 90 days prior to surgery. Open operations included any procedure performed in open fashion, including planned open operations and conversion to open. Pancreatic duct size and gland texture were classified according to NSQIP-defined variables. Intermediate gland texture and duct size 3 to 6 mm were used as a reference group in multivariable analysis.
All outcomes were measured for the 30-day post-operative period. Wound complications were defined as any superficial, deep incisional, or organ space infection. A pancreatic fistula was defined according to NSQIP definition and included those patients with persistent drainage of amylase-rich fluid (three times greater than serum level) beyond post-operative day 3 or clinical documentation by the attending surgeon of a pancreatic fistula. Delayed gastric emptying was defined by NSQIP as no oral intake beyond post-operative day 14 or reinsertion of nasogastric or gastrostomy tube to external drainage after post-operative day 7. Any percutaneous drain placed in the post-operative period was also documented.
Statistical Analysis
Univariate analysis was completed using Chi-square and Fisher exact test for categorical variables. The Student’s t test and Mann-Whitney U test were used according to the normalcy of data distribution. All demographic, operative, and outcome variables had less than 5% missing data with the exception of gland texture (27%) and duct size (25%). Multivariable regressions were used to account for patient demographics, comorbid conditions, and operative characteristics. Purposeful selection was used to select variables for inclusion in each model.15 This included all variables with P value < 0.01 on univariate analysis as well as those factors found to be predictive of each evaluated endpoint in previous studies. Hosmer-Lemeshow testing was used to confirm goodness of fit for each model. A P value < 0.05 was considered significant. All statistical analysis was performed using the SPSS statistical package (version 21.0 Armonk, NY). The institutional board for George Washington University Medical center approved this study and waived consent due to the de-identified nature of the data.
Results
We identified 3002 patients who underwent pancreaticoduodenectomy. A total of 436 (15%) patients underwent vascular reconstruction and 2566 (85%) did not. Among those undergoing reconstructions, 384 (88%) were venous and 52 (12%) were arterial and 81 (2.6%) underwent both.
Demographics and Comorbidities
Demographic characteristics and patient comorbidities are compared in Table 1. Patient age was similar among groups. Patients who did not undergo vascular reconstruction were more commonly male (54% vs. 47%, P < 0.01) than those who underwent venous reconstruction. There was no difference in sex between patients who underwent arterial reconstruction and those who underwent no reconstruction and those who underwent both compared to none. Patients who underwent both types of reconstruction were less often of white race (68% vs. 80%, P = 0.046) compared to those who did not undergo reconstruction. When comorbidities were assessed, patients who did not undergo reconstruction had lower rates of congestive heart failure than those who underwent venous reconstruction (0.2% vs. 1.8%, P < 0.01). Patients who did not undergo reconstruction also had less COPD (4.5% vs. 11.5%, P = 0.02) and a lower GFR (84 mg/dL vs. 87 mg/dL, P = 0.03) than those who underwent arterial reconstruction. Patients who underwent both types of reconstruction less often had diabetes compared to those who did not (17% vs. 25%, P < 0.01). Other comorbidities including smoking, hypertension, and chronic kidney disease were similar among groups.
Operative Characteristics
Operative characteristics are compared in Table 2. Malignancy was the primary pathology for pancreaticoduodenectomy in both groups; however, higher rates were seen in patients undergoing venous reconstruction compared to no vascular reconstruction (95% vs. 77%, P < 0.01) and in patients undergoing both venous and arterial reconstruction (87% vs. 77%, P < 0.01). Pre-operative chemotherapy was more common in venous (34% vs. 12%, P < 0.01), arterial (21% vs. 12%, P = 0.04), and both types of reconstruction (21% vs. 12%, P < 0.05) compared to no reconstruction. Also, compared to no reconstruction, patients who underwent venous reconstruction had higher rates of radiation (19% vs. 5.4%, P < 0.01) and preoperative stent placement (63% vs 51%, P < 0.01). Similarly, those who underwent both types of reconstruction also had higher rates of radiation (7.7% vs. 5.4%, P < 0.05). There were also significant differences in pancreatic duct size between those who underwent venous reconstruction and those who underwent no reconstruction (< 3 mm: 23% vs. 32%, 3–6 mm: 58% vs. 51%, > 6 mm: 19% vs. 17%; P = 0.01) and gland texture (hard: 59% vs. 41%, intermediate 12% vs. 9%, soft: 28% vs. 50%; P < 0.01). Patients who underwent both types of reconstruction also had differences in gland texture (hard: 49% vs. 41%, intermediate: 14% vs. 9%, soft: 37% vs. 50%, P < 0.05) and more often were noted to use an invagination technique (27% vs 11%, P < 0.01). Finally, vascular reconstruction was associated with increased median operative times following venous (441 min vs. 350 min, P < 0.01) and arterial reconstruction (431 min vs. 350 min, P < 0.01).
Outcomes
There was no difference in 30-day mortality (2.5% vs. 2.3%) or any major morbidities between patients who did not undergo any vascular reconstruction and those who underwent venous reconstruction in either univariate or multivariable analysis (Table 3). However, compared to patients who did not undergo any reconstruction, arterial reconstruction was associated with increased 30-day mortality (odds ratio (OR): 6.7, 95% confidence interval (CI): 1.8–25). Additionally, return to the operating room (OR: 4.5, 95% CI: 1.5–15), reintubation (OR: 3.9, 95% CI: 1.1–14), and pancreatic fistula (OR: 4.4, 95% CI: 1.7–11) were more common following arterial reconstruction. All other morbidities including wound infection, pneumonia, thromboembolic events, myocardial infarction, percutaneous drain placement, and delayed gastric emptying were similar.
Discussion
These data suggest that venous reconstruction during pancreaticodudenectomy can be performed safely without an increase in 30-day morbidity or mortality despite longer operative times and more advanced disease. However, arterial reconstruction is associated with higher 30-day mortality and morbidity.
According to the NCCN Guidelines, borderline resectable tumors are defined as tumor contact with the superior mesenteric vein or portal vein greater than 180°, contact of less than or equal to 180° with contour irregularity of the vein or thrombosis of vein but with suitable vessel proximal or distal and contact with the inferior vena cava (IVC).6 The International Study Group of Pancreatic Surgery reports that venous reconstruction should be attempted if R0 resection is feasible and also be performed at high volume centers.7 We identified isolated venous reconstruction rates of 15% within our study that is comparable to venous reconstruction rates in the reported literature, which range from 6 to 17.6%.16,17,18 Multiple small studies have also reported equivalent short- and long-term outcomes related to portal vein and superior mesenteric vein reconstruction as compared to those with no reconstruction.17, 19 Martin et al. describe no difference in peri-operative death or complications including wound infections, readmissions, and pancreatic leaks; however, these studies were limited by sample size.17 To better assess the effect of venous reconstruction on perioperative outcomes, several meta-analyses have been performed.20, 21 In an analysis of 22 smaller studies, Yu et al. found that although venous reconstruction required longer operative time, morbidity and mortality were not significantly different between those who did and did not undergo reconstruction.21 However, these results differed from those obtained by Giovainazzo et al. who found that patients who underwent venous reconstruction had increased 30-day mortality (3.9% vs. 3.0%).16 These mortality rates are higher than those found in the current study. The differences in the results found by Giovainazzo et al. may be explained by several factors. It should be noted that the study by Giovainazzo et al. evaluated all pancreatic resections including total pancreatectomy, distal pancreatectomy, and pancreaticoduodenectomy, which may not be generalizable. Additionally, these analyses included studies published from 1996 through 2014 and may not be comparable to the outcomes of patients who underwent pancreaticoduodenectomy from 2014 to 2015 as in our study. Finally, the authors acknowledge that they were unable to adjust for the use of neoadjuvant therapies, which may have affected patient outcomes. Finally, in one of the largest studies on vascular reconstruction, A NSQIP analysis from 2005 to 2009 evaluated 3582 patients who underwent pancreaticoduodenectomy including 281 patients who underwent vascular reconstruction found vascular reconstruction to be associated with an increased 30-day mortality (5.7% vs. 2.9%).10 However, it should be noted that this study was unable to differentiate between venous and arterial reconstruction, which our study found to have a differential effect on perioperative morbidity and mortality. Additionally, the targeted NSQIP module was not available in 2009, and therefore, authors were unable to account for intraoperative differences including duct size, gland texture, and pathology, all of which have been shown to be predictive of adverse post-operative events.22,23,24,25,26,27
Our study found a 2% rate of arterial reconstruction during pancreaticoduodenectomy. Few studies have looked at the incidence of isolated arterial reconstruction in pancreaticoduodenectomy, but rates have been reported ranging from 0.2 to 7%.8, 17, 28 Many of these studies are limited to single institution studies with an extremely small study cohort and poorly defined short-term outcomes. Although rare, arterial involvement with subsequent reconstruction in pancreatic cancer has been associated with worse long-term survival. Additionally, studies have suggested arterial involvement to be an indicator of advanced stage tumor while venous extension may be solely due to tumor location.2, 28 Mollberg et al. found a median mortality rate of 12% in total of 291 patients as well as high rates of overall morbidity. These results differ from those found by Glebova et al. In a single institution review of 35 patients who underwent arterial reconstruction between 1970 and 2014, authors found no differences in perioperative complications including leak, bleeding, or wound complications; however, short-term mortality was not included.28
While our study cannot assess long-term outcomes, our short-term results from the NSQIP database are consistent with Mollberg’s meta-analysis. The exact cause of why short-term morbidity is increased in the setting of arterial tumor reconstruction remains unclear. Factors such as anastomotic or reconstructive technique and tumor extent, which are not elucidated in prior studies, may be a reason for not only worse survival but worse short-term outcomes as well.
Our data support the safety of venous reconstruction during pancreaticoduodenectomy, which can be part of the pre-operative and surgical planning. This is important for preoperative counseling regarding potential risks of surgery as well as intraoperatively, where surgeons must balance the importance of R0 resections with the potential for post-operative morbidity. While we were only able to assess 52 patients with arterial reconstructions, the increased risk of 30-day mortality and morbidity are pertinent to account for in pre-operative planning and patient counseling. Surgeons should proceed with caution and consider highly skilled centers of excellence prior to arterial reconstruction.
Limitations
This study has limitations that must be noted. First, although it is subject to generic limitations of all large database analyses including errors in coding, missing data, and limited variable definitions, it should be noted that NSQIP is widely audited to ensure the quality and accuracy of data. The targeted pancreas module of NSQIP does not delineate the specific artery and vein (e.g., celiac artery vs. hepatic artery or portal vein vs. superior mesenteric vein) reconstructed. Therefore, we are unable to comment on differences in perioperative outcomes as they are influenced by reconstruction of specific vascular structures or conduit; furthermore, reconstruction technique and conduit are unknown. Similarly, while vascular involvement typically signifies locally advanced tumors, NSQIP neither provides information on the degree of perivascular involvement determined by preoperatively radiographic findings or intraoperative features, nor does it provide pathological findings regarding the degree of vascular invasion. As such, there may be a selection bias towards reconstructions with more favorable intraoperative or radiographic features that would confound our results. Additionally, the NSQIP database does not account for hospital volume or surgeon identifiers to distinguish if type of center affects outcomes. Since results of this study reflect 30-day outcomes, we are also unable to comment on long-term results, which is pertinent to oncological outcomes including disease-free or overall survival.
Conclusion
Pancreaticoduodenectomy with venous reconstruction is not associated with worse 30-day mortality or morbidity and may be safely performed despite a longer operative time. However, arterial reconstruction is associated with increased rates of adverse events including mortality. Therefore, careful consideration should be given prior to arterial reconstruction, while venous reconstruction should be performed in appropriate patients to achieve rates of R0 resection.
References
Christians KK, Heimler JW, George B, et al. Survival of patients with resectable pancreatic cancer who received neoadjuvant therapy. Surgery. 2016;159(3):893–900. doi:https://doi.org/10.1016/j.surg.2015.09.018
Mollberg N, Rahbari NN, Koch, et al. Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta-analysis. Annals of Surgery. 2011; 254(6), 882–893. https://doi.org/10.1097/SLA.0b013e31823ac299
Tummala P, Howard, T, Agarwal B. Dramatic Survival Benefit Related to R0 Resection of Pancreatic Adenocarcinoma in Patients With Tumor </=25 mm in Size and </=1 Involved Lymph Nodes. Clinical and Translational Gastroenterology. 2013;4,e33. https://doi.org/10.1038/ctg.2013.4
Evans DB, Farnell MB, Lillemoe KD, Vollmer C, Strasberg SM, Schulick RD. Surgical treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16(7):1736–1744. doi:https://doi.org/10.1245/s10434-009-0416-6.
Siriwardana HPP, Siriwardena AK. Systematic review of outcome of synchronous portal-superior mesenteric vein resection during pancreatectomy for cancer. Br J Surg. 2006;93(6):662–673. doi:https://doi.org/10.1002/bjs.5368.
Network NCCN. Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma Version 2.2017. 2017 [cited 2018 14 April]; Available from: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf.
Bockhorn M, Uzunoglu FG, Adham M, et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2014;155(6):977–88.
Hoshimoto S, Hishinuma S, Shirakawa H, et al. Reassessment of the clinical significance of portal-superior mesenteric vein invasion in borderline resectable pancreatic cancer. Eur J Surg Oncol. 2017;43(6):1068–75.
Worni M, Castleberry AW, Clary BM, et al. Concomitant vascular reconstruction during pancreatectomy for malignant disease: a propensity score-adjusted, population-based trend analysis involving 10,206 patients. JAMA Surgery. 2013;148(4):331–8.
Castleberry AW, White RR, De La Fuente SG, et al. The impact of vascular resection on early postoperative outcomes after pancreaticoduodenectomy: an analysis of the American College of Surgeons National Surgical Quality Improvement Program database. Ann Surg Oncol. 2012;19(13):4068–77.
Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–54.
Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137–47.
KDIGO 2012 clinical practice guidelines for the evaluation and management of chronic kidney disase. Kidney inter. 2012;2:1–138
Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical Care. 2007;11(2), R31. https://doi.org/10.1186/cc5713
Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. doi:https://doi.org/10.1186/1751-0473-3-17.
Giovinazzo F, Turri G, Katz MH, Heaton N, Ahmed I. Meta-analysis of benefits of portal-superior mesenteric vein resection in pancreatic resection for ductal adenocarcinoma. Br J Surg. 2016;103(3):179–191. doi:https://doi.org/10.1002/bjs.9969.
Martin RCG, Scoggins CR, Egnatashvili V, Staley CA, McMasters KM, Kooby DA. Arterial and venous resection for pancreatic adenocarcinoma: operative and long-term outcomes. Arch Surg Chic Ill 1960. 2009;144(2):154–159. doi:https://doi.org/10.1001/archsurg.2008.547.
Kelly KJ, Winslow E, Kooby D, et al. Vein involvement during pancreaticoduodenectomy: is there a need for redefinition of “borderline resectable disease”? J Gastrointest Surg Off J Soc Surg Aliment Tract. 2013;17(7):1209–1217; discussion 1217. doi:https://doi.org/10.1007/s11605-013-2178-5
Riediger H, Makowiec F, Fischer E, Adam U, Hopt UT. Postoperative morbidity and long-term survival after pancreaticoduodenectomy with superior mesenterico-portal vein resection. J Gastrointest Surg Off J Soc Surg Aliment Tract. 2006;10(8):1106–1115. doi:https://doi.org/10.1016/j.gassur.2006.04.002.
Ramacciato G, Mercantini P, Petrucciani N, et al. Does portal-superior mesenteric vein invasion still indicate irresectability for pancreatic carcinoma? Ann Surg Oncol. 2009;16(4):817–825. doi:https://doi.org/10.1245/s10434-008-0281-8.
Yu XZ, Li J, Fu DL, et al. Benefit from synchronous portal-superior mesenteric vein resection during pancreaticoduodenectomy for cancer: a meta-analysis. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2014;40(4):371–378. doi:https://doi.org/10.1016/j.ejso.2014.01.010
Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer Jr CM. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. Journal of the American College of Surgeons. 2013;216:114.
Greenblatt DY, Kelly KJ, Rajamanickam V, et al. Preoperative Factors Predict Perioperative Morbidity and Mortality After Pancreaticoduodenectomy. Annals of Surgical Oncology. 2011;18(8), 2126–2135. https://doi.org/10.1245/s10434-011-1594-6
Sandini M, Malleo G, Gianotti L. Scores for Prediction of Fistula after Pancreatoduodenectomy: A Systematic Review. Digestive Surgery. 2016;33(5), 392–400. https://doi.org/10.1159/000445068
Hu BY, Wan T, Zhang WZ, Dong JH. Risk factors for postoperative pancreatic fistula: analysis of 539 successive cases of pancreaticoduodenectomy. World Journal of Gastroenterology. 2016;22:7797–805.
Graham JA, Kayser R, Smirniotopoulos J, Nusbaum JD, Johnson LB. Probability prediction of a postoperative pancreatic fistula after a pancreaticoduodenectomy allows for more transparency with patients and can facilitate management of expectations. Journal of Surgical Oncology. 2013;108:137–8.
Kawai M, Kondo S, Yamaue H et al. Predictive risk factors for clinically relevant pancreatic fistula analyzed in 1239 patients with pancreaticoduodenectomy: multicenter data collection as a project study of pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. Journal of Hepato-Biliary-Pancreatic Sciences. 2011;18:601–8.
Glebova NO, Hicks CW, Tosoian JJ, et al. Outcomes of arterial resection during pancreatectomy for tumor. J Vasc Surg. 2016;63(3):722–729.e1. doi:https://doi.org/10.1016/j.jvs.2015.09.042
Acknowledgments
All authors have contributed significantly to this paper and meet the authorship guidelines as per the guidelines of the International Committee of Medical Journal Editors (ICMJE).
Author information
Authors and Affiliations
Corresponding author
Additional information
All authors listed above meet the authorship guidelines as per the guidelines of the International Committee of Medical Journal Editors (ICMJE).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zettervall, S.L., Ju, T., Holzmacher, J.L. et al. Arterial, but Not Venous, Reconstruction Increases 30-Day Morbidity and Mortality in Pancreaticoduodenectomy. J Gastrointest Surg 24, 578–584 (2020). https://doi.org/10.1007/s11605-019-04211-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-019-04211-2