Abstract
Background
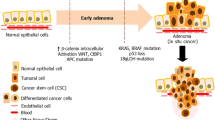
Colorectal cancer remains the most common gastrointestinal cancer. While screening combined with effective surgical treatment has reduced its mortality, we still do not have effective means to prevent recurrence nor to treat metastatic disease. What we know about cancer biology has gone through revolutionary changes in recent decades. The advent of the cancer stem cell theory has accelerated our understanding of the cancer cell. However, there is increasing evidence that cancer cells are influenced by their surrounding microenvironment.
Purpose
This review divides the tumor microenvironment into four functional components—the stem cell niche, cancer stroma, immune cells, and vascular endothelia—and examines their individual and collective influence on the growth and metastasis of the colon cancer stem cell. The discussion will highlight the need to fully exploit the tumor microenvironment when designing future prognostic tools and therapies.




Similar content being viewed by others
References
Cancer facts & Figures 2013, 2013, American Cancer Society: Atlanta.
Reya T and Clevers H. Wnt signalling in stem cells and cancer. Nature 2005;434:843-850.
Clevers H. The cancer stem cell: Premises, promises and challenges. Nature medicine 2011;17:313-319.
Kelly P, Dakic A, Adams J, Nutt S, and Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science (New York, N.Y.) 2007;317:337.
Gerlinger M, Rowan A, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, McDonald N, Butler A, Jones D, Raine K, Latimer C, Santos C, Nohadani M, Eklund A, Spencer-Dene B, Clark G, Pickering L, Stamp G, Gore M, Szallasi Z, Downward J, Futreal P, and Swanton C. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. The New England journal of medicine 2012;366:883-892.
Paget S. The distribution of secondary growths in cancer of the breast. The Lancet 1889;133:571-573.
Fidler I. The pathogenesis of cancer metastasis: The 'seed and soil' hypothesis revisited. Nature reviews. Cancer 2003;3:453-458.
Egeblad M, Nakasone E, and Werb Z. Tumors as organs: Complex tissues that interface with the entire organism. Developmental cell 2010;18:884-901.
Sato T, van Es J, Snippert H, Stange D, Vries R, van den Born M, Barker N, Shroyer N, van de Wetering M, and Clevers H. Paneth cells constitute the niche for lgr5 stem cells in intestinal crypts. Nature 2011;469:415-418.
Clevers H and Bevins C. Paneth cells: Maestros of the small intestinal crypts. Annual review of physiology 2013;75:289-311.
Krishnamurthy S, Dong Z, Vodopyanov D, Imai A, Helman J, Prince M, Wicha M, and Nör J. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer research 2010;70:9969-9978.
Jia L, Xiangcang Y, Fan F, Ling X, Rajat B, Seth B, Federico T, Eric S, Yunfei Z, Isamu T, Dipen MM, David HH, Janusz R, Sendurai AM, Patrick Z-M, and Lee ME. Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of jagged-1. Cancer cell 2013;23:171-185.
Rønnov-Jessen L, Petersen O, and Bissell M. Cellular changes involved in conversion of normal to malignant breast: Importance of the stromal reaction. Physiological reviews 1996;76:69-125.
Tlsty T and Hein P. Know thy neighbor: Stromal cells can contribute oncogenic signals. Current opinion in genetics & development 2001;11:54-59.
Jotzu C, Alt E, Welte G, Li J, Hennessy B, Devarajan E, Krishnappa S, Pinilla S, Droll L, and Song Y-H. Adipose tissue-derived stem cells differentiate into carcinoma-associated fibroblast-like cells under the influence of tumor-derived factors. Analytical cellular pathology (Amsterdam) 2010;33:61-79.
Mink S, Vashistha S, Zhang W, Hodge A, Agus D, and Jain A. Cancer-associated fibroblasts derived from egfr-tki-resistant tumors reverse egfr pathway inhibition by egfr-tkis. Molecular cancer research : MCR 2010;8:809-820.
Zeisberg E, Potenta S, Xie L, Zeisberg M, and Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer research 2007;67:10123-10128.
Bhowmick N, Chytil A, Plieth D, Gorska A, Dumont N, Shappell S, Washington M, Neilson E, and Moses H. Tgf-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science (New York, N.Y.) 2004;303:848-851
Kuperwasser C, Chavarria T, Wu M, Magrane G, Gray J, Carey L, Richardson A, and Weinberg R. Reconstruction of functionally normal and malignant human breast tissues in mice. Proceedings of the National Academy of Sciences of the United States of America 2004;101:4966-4971.
Bhowmick N, Neilson E, and Moses H. Stromal fibroblasts in cancer initiation and progression. Nature 2004;432:332-337.
Bierie B and Moses H. Tumour microenvironment: Tgfbeta: The molecular jekyll and hyde of cancer. Nature reviews. Cancer 2006;6:506-520.
Mishra L, Derynck R, and Mishra B. Transforming growth factor-beta signaling in stem cells and cancer. Science (New York, N.Y.) 2005;310:68-71
Siegel P and Massagué J. Cytostatic and apoptotic actions of tgf-beta in homeostasis and cancer. Nature reviews. Cancer 2003;3:807-821.
Zeisberg M, Strutz F, and Müller G. Role of fibroblast activation in inducing interstitial fibrosis. Journal of nephrology 2000;13 Suppl 3:S111-120.
Becker C, Fantini M, Schramm C, Lehr H, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, Ito H, Nishimoto N, Yoshizaki K, Kishimoto T, Galle P, Blessing M, Rose-John S, and Neurath M. Tgf-beta suppresses tumor progression in colon cancer by inhibition of il-6 trans-signaling. Immunity 2004;21:491-501.
Markowitz S, Wang J, Myeroff L, Parsons R, Sun L, Lutterbaugh J, Fan R, Zborowska E, Kinzler K, and Vogelstein B. Inactivation of the type ii tgf-beta receptor in colon cancer cells with microsatellite instability. Science (New York, N.Y.) 1995;268:1336-1338
Biswas S, Trobridge P, Romero-Gallo J, Billheimer D, Myeroff L, Willson J, Markowitz S, and Grady W. Mutational inactivation of tgfbr2 in microsatellite unstable colon cancer arises from the cooperation of genomic instability and the clonal outgrowth of transforming growth factor beta resistant cells. Genes, chromosomes & cancer 2008;47:95-106.
Biswas S, Chytil A, Washington K, Romero-Gallo J, Gorska A, Wirth P, Gautam S, Moses H, and Grady W. Transforming growth factor beta receptor type ii inactivation promotes the establishment and progression of colon cancer. Cancer research 2004;64:4687-4692.
Vermeulen L, De Sousa E Melo F, van der Heijden M, Cameron K, de Jong J, Borovski T, Tuynman J, Todaro M, Merz C, Rodermond H, Sprick M, Kemper K, Richel D, Stassi G, and Medema J. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nature cell biology 2010;12:468-476.
Cheng N, Bhowmick N, Chytil A, Gorksa A, Brown K, Muraoka R, Arteaga C, Neilson E, Hayward S, and Moses H. Loss of tgf-beta type ii receptor in fibroblasts promotes mammary carcinoma growth and invasion through upregulation of tgf-alpha-, msp- and hgf-mediated signaling networks. Oncogene 2005;24:5053-5068.
Waldner M, Foersch S, and Neurath M. Interleukin-6--a key regulator of colorectal cancer development. International journal of biological sciences 2012;8:1248-1253.
Waugh DJ and Wilson C. The interleukin-8 pathway in cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2008;14:6735-6741.
Korkaya H, Liu S, and Wicha M. Regulation of cancer stem cells by cytokine networks: Attacking cancer's inflammatory roots. Clinical cancer research : an official journal of the American Association for Cancer Research 2011;17:6125-6129.
Carpentino J, Hynes M, Appelman H, Zheng T, Steindler D, Scott E, and Huang E. Aldehyde dehydrogenase-expressing colon stem cells contribute to tumorigenesis in the transition from colitis to cancer. Cancer research 2009;69:8208-8215.
Guo Y, Xu F, Lu T, Duan Z, and Zhang Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer treatment reviews 2012;38:904-910.
Varney M, Singh S, Li A, Mayer-Ezell R, Bond R, and Singh R. Small molecule antagonists for cxcr2 and cxcr1 inhibit human colon cancer liver metastases. Cancer letters 2011;300:180-188.
Ginestier C, Liu S, Diebel M, Korkaya H, Luo M, Brown M, Wicinski J, Cabaud O, Charafe-Jauffret E, Birnbaum D, Guan J-L, Dontu G, and Wicha M. Cxcr1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. The Journal of clinical investigation 2010;120:485-497.
Otranto M, Sarrazy V, Bonté F, Hinz B, Gabbiani G, and Desmoulière A. The role of the myofibroblast in tumor stroma remodeling. Cell adhesion & migration 2012;6:203-219.
De Wever O, Demetter P, Mareel M, and Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. International journal of cancer. Journal international du cancer 2008;123:2229-2238.
Tse J, Cheng G, Tyrrell J, Wilcox-Adelman S, Boucher Y, Jain R, and Munn L. Mechanical compression drives cancer cells toward invasive phenotype. Proceedings of the National Academy of Sciences of the United States of America 2012;109:911-916.
Tsujino T, Seshimo I, Yamamoto H, Ngan C, Ezumi K, Takemasa I, Ikeda M, Sekimoto M, Matsuura N, and Monden M. Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2007;13:2082-2090.
Koukourakis M, Giatromanolaki A, Harris A, and Sivridis E. Comparison of metabolic pathways between cancer cells and stromal cells in colorectal carcinomas: A metabolic survival role for tumor-associated stroma. Cancer research 2006;66:632-637.
Balkwill F and Mantovani A. Inflammation and cancer: Back to virchow? Lancet 2001;357:539-545.
Coussens L and Werb Z. Inflammation and cancer. Nature 2002;420:860-867.
Houghton A, Rzymkiewicz D, Ji H, Gregory A, Egea E, Metz H, Stolz D, Land S, Marconcini L, Kliment C, Jenkins K, Beaulieu K, Mouded M, Frank S, Wong K, and Shapiro S. Neutrophil elastase-mediated degradation of irs-1 accelerates lung tumor growth. Nature medicine 2010;16:219-223.
Nozawa H, Chiu C, and Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America 2006;103:12493-12498.
Ong S-M, Tan Y-C, Beretta O, Jiang D, Yeap W-H, Tai J, Wong W-C, Yang H, Schwarz H, Lim K-H, Koh P-K, Ling K-L, and Wong S-C. Macrophages in human colorectal cancer are pro-inflammatory and prime t cells towards an anti-tumour type-1 inflammatory response. European journal of immunology 2012;42:89-100.
Edin S, Wikberg M, Rutegård J, and Oldenborg… P. Phenotypic skewing of macrophages in vitro by secreted factors from colorectal cancer cells. PloS one 2013;8:e74982
Erreni M, Mantovani A, and Allavena P. Tumor-associated macrophages (tam) and inflammation in colorectal cancer. Cancer microenvironment : official journal of the International Cancer Microenvironment Society 2011;4:141-154.
Nakanishi Y, Nakatsuji M, Seno H, Ishizu S, Akitake-Kawano R, Kanda K, Ueo T, Komekado H, Kawada M, Minami M, and Chiba T. Cox-2 inhibition alters the phenotype of tumor-associated macrophages from m2 to m1 in apcmin/+ mouse polyps. Carcinogenesis 2011;32:1333-1339.
Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, and Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2000;6:1755-1766.
Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nature reviews. Immunology 2004;4:941-952.
Novitskiy SV, Ryzhov S, Zaynagetdinov R, Goldstein AE, Huang Y, Tikhomirov OY, Blackburn MR, Biaggioni I, Carbone DP, Feoktistov I, and Dikov MM. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood 2008;112:1822-1831.
Gabrilovich D, Ostrand-Rosenberg S, and Bronte V. Coordinated regulation of myeloid cells by tumours. Nature reviews. Immunology 2012;12:253-268.
Shojaei F, Wu X, Qu X, Kowanetz M, Yu L, Tan M, Meng Y, and Ferrara N. G-csf-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-vegf therapy in mouse models. Proceedings of the National Academy of Sciences of the United States of America 2009;106:6742-6747.
Pylayeva-Gupta Y, Lee K, Hajdu C, Miller G, and Bar-Sagi D. Oncogenic kras-induced gm-csf production promotes the development of pancreatic neoplasia. Cancer cell 2012;21:836-847.
Motz G and Coukos G. The parallel lives of angiogenesis and immunosuppression: Cancer and other tales. Nature reviews. Immunology 2011;11:702-711.
Pardoll D. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews. Cancer 2012;12:252-264.
Di Renzo M, Olivero M, Giacomini A, Porte H, Chastre E, Mirossay L, Nordlinger B, Bretti S, Bottardi S, and Giordano S. Overexpression and amplification of the met/hgf receptor gene during the progression of colorectal cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 1995;1:147-154.
Mao Q, Zhang Y, Fu X, Xue J, Guo W, Meng M, Zhou Z, Mo X, and Lu Y. A tumor hypoxic niche protects human colon cancer stem cells from chemotherapy. Journal of cancer research and clinical oncology 2013;139:211-222.
Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, Liu JK, Fang X, Sloan AE, Mao Y, Lathia JD, Min W, McLendon RE, Rich JN, and Bao S. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell 2013;153:139-152.
Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature medicine 1995;1:27-31.
Cook K and Figg W. Angiogenesis inhibitors: Current strategies and future prospects. CA: a cancer journal for clinicians 2010;60:222-243.
Shih T and Lindley C. Bevacizumab: An angiogenesis inhibitor for the treatment of solid malignancies. Clinical therapeutics 2006;28:1779-1802.
Siemann D. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by tumor-vascular disrupting agents. Cancer treatment reviews 2011;37:63-74.
Acknowledgments
We gratefully acknowledge Kristen Huang, PhD, for careful reading of the text.
Author information
Authors and Affiliations
Corresponding author
Additional information
Grant Support: Sugong Chen receives support from NIH T32 CA106493, awarded to Kevin E Behrns. Emina H. Huang is the principal investigator of NIH R01 CA142808 and R01 CA157663.
Rights and permissions
About this article
Cite this article
Chen, S., Huang, E.H. The Colon Cancer Stem Cell Microenvironment Holds Keys to Future Cancer Therapy. J Gastrointest Surg 18, 1040–1048 (2014). https://doi.org/10.1007/s11605-014-2497-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-014-2497-1