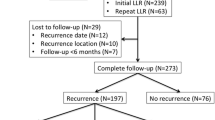
Abstract
Aim
To assess the impact of first recurrence location on survival following surgery of colorectal liver metastases.
Methods
A total of 265 consecutive patients with colorectal liver metastases undergoing liver surgery (2000–2011) were categorized according to first site of tumor recurrence. Time to recurrence (TTR) and overall survival (OS) were determined. Uni- and multivariate analysis were performed to identify factors associated with TTR and OS.
Results
Median TTR was 1.16 years following liver resection, and 0.56 years following radiofrequency ablation (RFA). Intrahepatic recurrence following liver resection resulted in a significantly shorter median TTR compared to extrahepatic recurrence. Intrapulmonary recurrence was associated with superior survival compared to other recurrence locations. Such patterns were not observed in the RFA-treated group. Multivariate analysis identified the type of surgical treatment and extra-hepatic first-site recurrence (other than lung) as independent predictors for OS. Pre-operative chemotherapy and simultaneous intrahepatic and extrahepatic recurrence were independent predictors for both TTR and OS.
Conclusions
Patients with intrahepatic recurrence following liver resection have a significantly shorter TTR and OS when compared to patients developing extrahepatic recurrence. Pulmonary recurrence following resection is associated with longer survival. Simultaneous intra- and extrahepatic recurrence is an independent prognostic factor for TTR and OS.

Similar content being viewed by others
References
Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Annals of Surgery 2006 August;244(2):254–9.
Otto G, Duber C, Hoppe-Lotichius M, Konig J, Heise M, Pitton MB. Radiofrequency ablation as first-line treatment in patients with early colorectal liver metastases amenable to surgery. Annals of Surgery 2010 May;251(5):796–803.
Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. British Journal of Cancer 2006 April 10;94(7):982–99.
Pathak S, Jones R, Tang JM, Parmar C, Fenwick S, Malik H, Poston G. Ablative therapies for colorectal liver metastases: a systematic review. Colorectal Disease 2011 September;13(9):e252–e265.
Hur H, Ko YT, Min BS, Kim KS, Choi JS, Sohn SK, Cho CH, Ko HK, Lee JT, Kim NK. Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal liver metastases. American Journal of Surgery 2009 June;197(6):728–36.
Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, Hess K, Curley SA. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Annals of Surgery 2004 June;239(6):818–25.
White RR, Avital I, Sofocleous CT, Brown KT, Brody LA, Covey A, Getrajdman GI, Jarnagin WR, Dematteo RP, Fong Y, Blumgart LH, D'Angelica M. Rates and patterns of recurrence for percutaneous radiofrequency ablation and open wedge resection for solitary colorectal liver metastasis. Journal of Gastrointestinal Surgery 2007 March;11(3):256–63.
Gleisner AL, Choti MA, Assumpcao L, Nathan H, Schulick RD, Pawlik TM. Colorectal liver metastases: recurrence and survival following hepatic resection, radiofrequency ablation, and combined resection–radiofrequency ablation. Archives of Surgery 2008 December;143(12):1204–12.
de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, Choti MA, Aldrighetti L, Capussotti L, Pawlik TM. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Annals of Surgery 2009 September;250(3):440–8.
D'Angelica M, Kornprat P, Gonen M, Dematteo RP, Fong Y, Blumgart LH, Jarnagin WR. Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Annals of Surgery Oncology 2011 April;18(4):1096–103.
Assumpcao L, Choti MA, Gleisner AL, Schulick RD, Swartz M, Herman J, Gearhart SL, Pawlik TM. Patterns of recurrence following liver resection for colorectal metastases: effect of primary rectal tumor site. Archives of Surgery 2008 August;143(8):743–9.
Kingham TP, Tanoue M, Eaton A, Rocha FG, Do R, Allen P, De Matteo RP, D'Angelica M, Fong Y, Jarnagin WR. Patterns of recurrence after ablation of colorectal cancer liver metastases. Annals of Surgery Oncology 2011 August 31;19(3):834–41.
Andreou A, Brouquet A, Abdalla EK, Aloia TA, Curley SA, Vauthey JN. Repeat hepatectomy for recurrent colorectal liver metastases is associated with a high survival rate. HPB (Oxford) 2011 November;13(11):774–82.
Bismuth H, Adam R, Navarro F, Castaing D, Engerran L, Abascal A. Re-resection for colorectal liver metastasis. Surgical Oncology Clinics of North America 1996 April;5(2):353–64.
Koopman M, Antonini NF, Douma J, Wals J, Honkoop AH, Erdkamp FL, de Jong RS, Rodenburg CJ, Vreugdenhil G, Loosveld OJ, van BA, Sinnige HA, Creemers GJ, Tesselaar ME, Slee PH, Werter MJ, Mol L, Dalesio O, Punt CJ. Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomised controlled trial. Lancet 2007 July 14;370(9582):135–42.
Cosimelli M, Golfieri R, Cagol PP, Carpanese L, Sciuto R, Maini CL, Mancini R, Sperduti I, Pizzi G, Diodoro MG, Perrone M, Giampalma E, Angelelli B, Fiore F, Lastoria S, Bacchetti S, Gasperini D, Geatti O, Izzo F. Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. British Journal of Cancer 2010 July 27;103(3):324–31.
Martin LK, Cucci A, Wei L, Rose J, Blazer M, Schmidt C, Khabiri H, Bloomston M, Bekaii-Saab T. Yttrium-90 radioembolization as salvage therapy for colorectal cancer with liver metastases. Clinical Colorectal Cancer 2012 January 23.
Nanko M, Shimada H, Yamaoka H, Tanaka K, Masui H, Matsuo K, Ike H, Oki S, Hara M. Micrometastatic colorectal cancer lesions in the liver. Surgery Today 1998;28(7):707–13.
Wakai T, Shirai Y, Sakata J, Valera VA, Korita PV, Akazawa K, Ajioka Y, Hatakeyama K. Appraisal of 1 cm hepatectomy margins for intrahepatic micrometastases in patients with colorectal carcinoma liver metastasis. Annals of Surgery Oncology 2008 September;15(9):2472–81.
Yokoyama N, Shirai Y, Ajioka Y, Nagakura S, Suda T, Hatakeyama K. Immunohistochemically detected hepatic micrometastases predict a high risk of intrahepatic recurrence after resection of colorectal carcinoma liver metastases. Cancer 2002 March 15;94(6):1642–7.
Bismuth H. Surgical anatomy and anatomical surgery of the liver. World Journal of Surgery 1982 January;6(1):3–9.
Polissar L, Diehr P. Regression analysis in health services research: the use of dummy variables. Medical Care 1982 September;20(9):959–66.
Stoecklein NH, Klein CA. Genetic disparity between primary tumours, disseminated tumour cells, and manifest metastasis. International Journal of Cancer 2010 February 1;126(3):589–98.
Goasguen N, de CC, Brouquet A, Julie C, Prevost GP, Laurent-Puig P, Penna C. Evidence of heterogeneity within colorectal liver metastases for allelic losses, mRNA level expression and in vitro response to chemotherapeutic agents. International Journal of Cancer 2010 September 1;127(5):1028–37.
Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, Curley SA, Loyer EM, Muratore A, Mentha G, Capussotti L, Vauthey JN. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Annals of Surgery 2005 May;241(5):715–22, discussion.
Fernandez FG, Drebin JA, Linehan DC, Dehdashti F, Siegel BA, Strasberg SM. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET). Annals of Surgery 2004 September;240(3):438–47.
McKay A, Fradette K, Lipschitz J. Long-term outcomes following hepatic resection and radiofrequency ablation of colorectal liver metastases. HPB Surgery 2009;2009:346863.
Wong SL, Mangu PB, Choti MA, Crocenzi TS, Dodd GD, III, Dorfman GS, Eng C, Fong Y, Giusti AF, Lu D, Marsland TA, Michelson R, Poston GJ, Schrag D, Seidenfeld J, Benson AB, III. American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. Journal of Clinical Oncology 2010 January 20;28(3):493–508.
Reuter NP, Woodall CE, Scoggins CR, McMasters KM, Martin RC. Radiofrequency ablation vs. resection for hepatic colorectal metastasis: therapeutically equivalent? Journal of Gastrointestinal Surgery 2009 March;13(3):486–91.
Nijkamp MW, van der Bilt JD, de Bruijn MT, Molenaar IQ, Voest EE, van Diest PJ, Kranenburg O, Borel RIH. Accelerated perinecrotic outgrowth of colorectal liver metastases following radiofrequency ablation is a hypoxia-driven phenomenon. Annals of Surgery 2009 May;249(5):814–23.
Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nature Reviews Cancer 2008 December;8(12):967–75.
Klampfer L. Cytokines, inflammation and colon cancer. Current Cancer Drug Targets 2011 May;11(4):451–64.
Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends in Pharmacological Science 2012 April;33(4):207–14.
Govaert KM, Nijkamp MW, Emmink BL, Steller EJ, Minchinton AI, Kranenburg O, Borel R, I. Effects of tirapazamine on experimental colorectal liver metastases after radiofrequency ablation. British Journal of Surgery 2012 February 13;99(4):567–75.
Ahmad A, Chen SL, Kavanagh MA, Allegra DP, Bilchik AJ. Radiofrequency ablation of hepatic metastases from colorectal cancer: are newer generation probes better? American Surgeon 2006 October;72(10):875–9.
Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Collette L, Praet M, Bethe U, Van CE, Scheithauer W, Gruenberger T. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008 March 22;371(9617):1007–16.
Schnetzler B, Popova N, Collao LC, Sappino AP. Coronary spasm induced by capecitabine. Annals of Oncology 2001 May;12(5):723–4.
Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nature Reviews Cancer 2002 January;2(1):38–47.
Lu X, Kang Y. Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clinical Cancer Research 2010 December 15;16(24):5928–35.
Govaert KM, Emmink BL, Nijkamp MW, Cheung ZJ, Steller EJ, Fatrai S, de Bruijn MT, Kranenburg O, Borel R, I. Hypoxia after liver surgery imposes an aggressive cancer stem cell phenotype on residual tumor cells. Annals of Surgery 2013 November 18.
Acknowledgements
This study was supported by the Dutch Cancer Society (grant no. UU 2010-4608 to K.M.G., UU 2009 4367 to B.L.E. and UU 2009-4379 to E.J.A.S.) and the PON foundation (E.J.A.S.).
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
K.M. Govaert and C.S. van Kessel contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig2
Supplemental Figure 1 Kaplan Meijer curve of time to recurrence(TTR) (A) and overall survival(OS) (B) for the different recurrence categories following surgical treatment of colorectal liver metastases. (JPEG 35 kb)
(JPEG 34 kb)
11605_2014_2461_MOESM3_ESM.docx
Supplemental Table 1 Overall survival corrected for time to recurrence according to the different recurrence locations following surgical treatment of colorectal liver metastases. Values are given in medians with 95%CI. (RFA: radiofrequency ablation) (DOCX 11 kb)
11605_2014_2461_MOESM4_ESM.docx
Supplemental Table 2 Baseline characteristics of patients select for with or without neo adjuvant chemotherapy. Data is based on 262 patients (instead of 265) because data about neo adjuvant chemotherapy was lacking in 3 cases. When percentages are based on less than 263 patients, it is indicated. (RFA: radiofrequency ablation. EHD: extra hepatic disease; CRLM: colorectal liver metastases.) (DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Govaert, K.M., van Kessel, C.S., Steller, E.J.A. et al. Recurrence Location After Resection of Colorectal Liver Metastases Influences Prognosis. J Gastrointest Surg 18, 952–960 (2014). https://doi.org/10.1007/s11605-014-2461-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-014-2461-0