Abstract
Background
Duodenal neuroendocrine tumors are rare and few studies exist to guide surgical management. This study identifies factors associated with recurrence after resection.
Methods
A retrospective, single institution review was performed between 1983 and 2011 on patients with a pathologic diagnosis of duodenal neuroendocrine tumor. Tumor grade was assigned based on WHO 2010 criteria (Ki-67 and mitotic rate).
Results
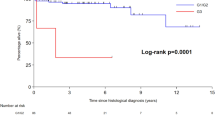
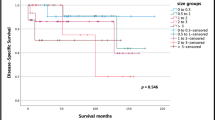
Seventy-five patients were identified that underwent curative resection. This included 12 patients with endoscopic mucosal resection, 34 that had local resection, and 29 that underwent pancreaticoduodenectomy. Two-year and 5-year recurrence-free survival was 84 and 81 %, respectively. There were 11 tumor recurrences (either local or distant), and four patients died of their disease (3/4 had high-grade lesions) with an overall median follow-up of 27 months. On univariate analysis, tumor size and tumor grade were identified as being associated with recurrence, but not intervention type, lymph node metastases, ampullary location, or margin status.
Conclusions
Tumor grade and size are associated with recurrence-free survival in duodenal neuroendocrine tumors. When feasible, a less aggressive surgical approach to treat low-grade and low-stage duodenal NETs should be considered.



Similar content being viewed by others
References
Albores-Saavedra J, Hart A, Chablé-Montero F, Henson DE. Carcinoids and high-grade neuroendocrine carcinomas of the ampulla of vater: a comparative analysis of 139 cases from the surveillance, epidemiology, and end results program-a population based study. Arch Pathol Lab Med 2010 ;134:1692–6.
Mullen JT, Wang H, Yao JC, Lee JH, Perrier ND, Pisters PW, Lee JE, Evans DB. Carcinoid tumors of the duodenum. Surgery 2005;138:971–7
Zyromski NJ, Kendrick ML, Nagorney DM, Grant CS, Donohue JH, Farnell MB, Thompson GB, Farley DR, Sarr MG. Duodenal carcinoid tumors: how aggressive should we be? J Gastrointest Surg 2001;5:588–93
Burke AP, Sobin LH, Federspiel BH, Shekitka KM, Helwig EB. Carcinoid tumors of the duodenum. A clinicopathologic study of 99 cases. Arch Pathol Lab Med 1990;114:700–4.
Mao C, Shah A, Hanson DJ, Howard JM. Von Recklinghausen’s disease associated with duodenal somatostatinoma: contrast of duodenal versus pancreatic somatostatinomas. J Surg Oncol 1995;59:67–73
Reidy-Lagunes D, Thornton R. Pancreatic neuroendocrine and carcinoid tumors: what’s new, what’s old, and what’s different? Curr Oncol Rep 2012;14:249–56.
Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas 2010;39:707–12.
Grobmyer SR, Pieracci FM, Allen PJ, Brennan MF, Jaques DP. Defining morbidity after pancreaticoduodenectomy: use of a prospective complication grading system. J Am Coll Surg 2007;204:356–64.
Yachida S, Vakiani E, White CM, Zhong Y, Saunders T, Morgan R, de Wilde RF, Maitra A, Hicks J, Demarzo AM, Shi C, Sharma R, Laheru D, Edil BH, Wolfgang CL, Schulick RD, Hruban RH, Tang LH, Klimstra DS, Iacobuzio-Donahue CA. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol 2012;36:173–84.
Nassar H, Albores-Saavedra J, Klimstra DS. High-grade neuroendocrine carcinoma of the ampulla of vater: a clinicopathologic and immunohistochemical analysis of 14 cases. Am J Surg Pathol 2005;29:588–94.
Makhlouf HR, Burke AP, Sobin LH. Carcinoid tumors of the ampulla of Vater: a comparison with duodenal carcinoid tumors. Cancer 1999;85:1241–9.
Suzuki S, Tanaka S, Hayashi T, Harada N, Suzuki M, Hanyu F, Ban S. Small-cell neuroendocrine carcinoma of the ampulla of Vater. J Hepatobiliary Pancreat Surg 2006;13:450–3.
Hartel M, Wente MN, Sido B, Friess H, Büchler MW. Carcinoid of the ampulla of Vater. J Gastroenterol Hepatol 2005;20:676–81.
Strosberg J, Nasir A, Coppola D, Wick M, Kvols L. Correlation between grade and prognosis in metastatic gastroenteropancreatic neuroendocrine tumors. Hum Pathol 2009;40:1262–8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Discussant
Dr. Charles Yeo (Philadelphia, PA): I rise to congratulate Dr. Untch and his coauthors from the Memorial Sloan-Kettering Cancer Center. This is another well-done retrospective review from this esteemed institution. The authors have given us additional information about these rare, duodenal neuroendocrine tumors. This is a laudable experience, and it significantly adds to the literature. Thank you very much for providing me with a draft of the manuscript prior to the talk today. I have several comments and then four specific questions for you to address.
First, as concerns my comments, I would ask you to underscore in your manuscript that the Zollinger-Ellison syndrome and the somatostatinoma syndrome are clinical definitions and note that they are not simply immunohistochemical findings based upon specific stains. This is certainly not clear in your manuscript. Second, to my reading, your univariate analysis for recurrence has significance with grade, size, and margin status, although the p value for the latter is only p = 0.06 and the hazard ratio is 3. I seriously doubt that this is a type 1 error. I would like to see, however, the multivariate analysis. Third, although you mention some limitations in your manuscript, there are some additional clear limitations to the study. Yes, this is a relatively small group of patients with this rare tumor. There clearly was some treatment bias as to whether the patient underwent endoscopic therapy, segmental resection, or more extensive regional resection. The experience extends over quite a long period of time, where treatment may have changed. Clearly, the patients who underwent endoscopic therapy are not at all matched to the patients who underwent more extensive resections, and again, there is no multivariate analysis. Fourth, your median follow-up is only 27 months, which is really quite short for patients with endocrine neoplasia. In fact, your follow-up was only 9 months in the endoscopic resection group. I would suggest that most statisticians would insist that you truncate your Kaplan-Meier survival curves at 5 years, as extending them out beyond more than twice the duration of your median follow-up is considered by some statisticians to be misleading.
I do have four short questions for you. First, can you provide substantive evidence to us that these were all duodenal endocrine tumors and not pancreatic endocrine tumors? Really, what I am asking is did one of the world’s experts at your institution David Klimstra, a well-known pathologist, reviewed the slides? Second, it is always curious to me how to apply the WHO criteria to tumor grading. The criteria involve the number of mitoses per 10 high-power fields, as well as the Ki67 immunostaining reported as a percentage. Which of these two criteria wins out? By this, I am asking if by the mitoses criteria, the tumor would be classified as an intermediate-grade tumor (2–10 mitoses per 10 high-power fields), while the Ki67 immunostaining shows less than 3 % of cells staining which would make it a low-grade tumor, which is it? Stated differently, do mitoses trump Ki67 or vice versa? Third, truly, how carefully did you look for recurrence? You are reporting to us disease-free survival. I would suggest that showing overall survival is perhaps more helpful, as the dichotomized variable alive versus dead is a more meaningful endpoint as compared to whether or not you have identified recurrence during follow-up. Fourth, how specifically do you use tumor grading to “personalize” postoperative care? Does tumor grading somehow modify the extent or aggressiveness of your follow-up? Does tumor grading somehow alter your decision to either offer or not offer postoperative adjuvant therapy?
Again, thank you for a well-presented paper and a fine manuscript.
Closing Discussant
Dr. Brian Untch: Thank you Dr. Yeo for reviewing the paper and for your comments. In regard to differentiating duodenal and pancreatic neuroendocrine tumors, pathology review was undertaken by two of the coauthors, Dr. Laura Tang and Dr. David Klimstra, both of whom have a specialized interest in neuroendocrine tumors. They have a long track record of studying these tumors having published numerous studies. I have complete confidence that we have identified only duodenal neuroendocrine tumors in this series.
Mitoses and KI-67 IHC were used to determine grade in our study according to WHO classification. The situation you describe of inconsistent mitoses/HPF and KI-67 labeling could occur and there has been consensus on this issue. Whenever the discrepancy occurs, the higher grade is elected by either mitoses or Ki67 labeling.
The issue of recurrence is a challenging one in neuroendocrine tumors, as you point out, because there can be extended disease-free survival. In this study, we chose to focus on recurrence rather than overall survival because recurrences were more frequent and can be a surrogate for disease severity. Overall survival can be difficult to interpret in the setting of neuroendocrine tumors as many patients can die from unrelated reasons. Much of our efforts for this study focused on whether patients had recurrence, including a review of the patients’ clinical status and all imaging studies. Given that these patients are followed by interval CT scans, this was the primary method for which recurrence was identified.
While no adjuvant treatment currently exists for low- and intermediate-grade neuroendocrine tumors outside of a clinical trial, identifying prognostic factors, as we do in this study, should set the stage when it becomes available. Hopefully, it will also encourage stratification within ongoing and upcoming trials. We will only routinely give adjuvant chemotherapy to patients with high-grade neuroendocrine carcinomas as these patients are at high risk of recurrence and death, as seen in our study. In terms of follow-up, we have not yet adopted a risk-based program, but this is an area of active study at our institution.
The paper was presented during a plenary session at the SSAT annual meeting in Orlando, FL, May 21st 2013.
Rights and permissions
About this article
Cite this article
Untch, B.R., Bonner, K.P., Roggin, K.K. et al. Pathologic Grade and Tumor Size are Associated with Recurrence-Free Survival in Patients with Duodenal Neuroendocrine Tumors. J Gastrointest Surg 18, 457–463 (2014). https://doi.org/10.1007/s11605-014-2456-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-014-2456-x