Abstract
Introduction
Apoptosis plays a critical role in the maintenance of gut mucosal epithelial homeostasis and is tightly regulated by numerous factors including intracellular Ca2+. Canonical transient receptor potential channel-1 (TRPC1) is expressed in intestinal epithelial cells (IECs) and functions as a store-operated Ca2+ channel. We have recently demonstrated that increased TRPC1 activity sensitizes IECs to apoptosis, but the upstream signaling initiating TRPC1 activation remains elusive. The novel protein, stromal interaction molecule 1 (STIM1), is shown to act as a store Ca2+ sensor, and it can rapidly translocate to the plasma membrane where it directly interacts with TRPC1. The current study determined whether STIM1 plays an important role in the regulation of IEC apoptosis by activating TRPC1 channel activity.
Methods
Studies were conducted in IEC-6 cells (derived from rat intestinal crypts) and stable TRPC1-transfected IECs (IEC-TRPC1). Apoptosis was induced by tumor necrosis factor-α (TNF-α)/cycloheximide (CHX), and intracellular free Ca2+ concentration ([Ca2+]cyt) was measured by fluorescence digital imaging analysis. Functions of STIM1 were investigated by specific siRNA (siSTIM1) and ectopic overexpression of the constitutively active STIM1 EF-hand mutants.
Results
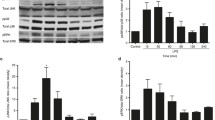
Stable STIM1-transfected IEC-6 cells (IEC-STIM1) showed increased STIM1 protein expression (~5 fold) and displayed a sustained increase in Ca2+ influx after Ca2+ store depletion (~2 fold). Susceptibility of IEC-STIM1 cells to TNF-α/CHX-induced apoptosis increased significantly as measured by changes in morphological features, DNA fragmentation, and caspase-3 activity. Apoptotic cells were increased from ~20% in parental IEC-6 cells to ~40% in stable IEC-STIM1 cells 4 h after exposure to TNF-α/CHX (p < 0.05). In addition, stable IEC-TRPC1 cells also exhibited an increased sensitivity to TNF-α/CHX-induced apoptosis, which was prevented by STIM1 silencing through siSTIM1 transfection. STIM1 silencing by siSTIM1 also decreased Ca2+ influx after store depletion in cells overexpressing TRPC1. Levels of Ca2+ influx due to store depletion were decreased by ~70% in STIM1-silenced populations. Similarly, exposure of IEC-STIM1 cells to Ca2+-free medium also blocked increased sensitivity to apoptosis.
Conclusions
These results indicate that (1) STIM1 plays an important role in the regulation of IEC apoptosis by altering TRPC1 activity and (2) ectopic STIM1 expression sensitizes IECs to apoptosis through induction in TRPC1-mediated Ca2+ influx.







Similar content being viewed by others
References
Booth, C., and Potten, C. S. (2000) Gut instincts: thoughts on intestinal epithelial stem cells, J Clin Invest 105, 1493–1499.
Potten, C. S. (1997) Epithelial cell growth and differentiation. II. Intestinal apoptosis, Am J Physiol 273, G253-257.
Orrenius, S., Zhivotovsky, B., and Nicotera, P. (2003) Regulation of cell death: the calcium-apoptosis link, Nat Rev Mol Cell Biol 4, 552–565.
Nicotera, P., and Orrenius, S. (1998) The role of calcium in apoptosis, Cell Calcium 23, 173–180.
Cho, C. H., and Wang, J. Y. (2002) Gastrointestinal mucosal repair and experimental therapeutics, Karger, Basel; New York.
Marasa, B. S., Rao, J. N., Zou, T., Liu, L., Keledjian, K. M., Zhang, A. H., Xiao, L., Chen, J., Turner, D. J., and Wang, J. Y. (2006) Induced TRPC1 expression sensitizes intestinal epithelial cells to apoptosis by inhibiting NF-kappaB activation through Ca2+ influx, Biochem J 397, 77–87.
Hewavitharana, T., Deng, X., Wang, Y., Ritchie, M. F., Girish, G. V., Soboloff, J., and Gill, D. L. (2008) Location and function of STIM1 in the activation of Ca2+ entry signals, J Biol Chem 283, 26252–26262.
Hewavitharana, T., Deng, X., Soboloff, J., and Gill, D. L. (2007) Role of STIM and Orai proteins in the store-operated calcium signaling pathway, Cell Calcium 42, 173–182.
Ong, H. L., Liu, X., Tsaneva-Atanasova, K., Singh, B. B., Bandyopadhyay, B. C., Swaim, W. D., Russell, J. T., Hegde, R. S., Sherman, A., and Ambudkar, I. S. (2007) Relocalization of STIM1 for activation of store-operated Ca(2+) entry is determined by the depletion of subplasma membrane endoplasmic reticulum Ca(2+) store, J Biol Chem 282, 12176–12185.
Quaroni, A., Wands, J., Trelstad, R. L., and Isselbacher, K. J. (1979) Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria, J Cell Biol 80, 248–265.
Rao, J. N., Rathor, N., Zou, T., Liu, L., Xiao, L., Yu, T. X., Cui, Y. H., and Wang, J. Y. (2010) STIM1 translocation to the plasma membrane enhances intestinal epithelial restitution by including TRPC1-medaited Ca2+ signaling after wounding, Am J Physiol 229, C579–588.
Worley, P. F., Zeng, W., Huang, G. N., Yuan, J. P., Kim, J. Y., Lee, M. G., and Muallem, S. (2007) TRPC channels as STIM1-regulated store-operated channels, Cell Calcium 42, 205–211.
Rao, J. N., Platoshyn, O., Golovina, V. A., Liu, L., Zou, T., Marasa, B. S., Turner, D. J., Yuan, J. X., and Wang, J. Y. (2006) TRPC1 functions as a store-operated Ca2+ channel in intestinal epithelial cells and regulates early mucosal restitution after wounding, Am J Physiol Gastrointest Liver Physiol 290, G782-792.
Marasa, B. S., Xiao, L., Rao, J. N., Zou, T., Liu, L., Wang, J., Bellavance, E., Turner, D. J., and Wang, J. Y. (2008) Induced TRPC1 expression increases protein phosphatase 2A sensitizing intestinal epithelial cells to apoptosis through inhibition of NF-kappaB activation, Am J Physiol Cell Physiol 294, C1277-1287.
Putney, J. W., Jr. (2005) Capacitative calcium entry: sensing the calcium stores, J Cell Biol 169, 381–382.
Zhang, S. L., Yu, Y., Roos, J., Kozak, J. A., Deerinck, T. J., Ellisman, M. H., Stauderman, K. A., and Cahalan, M. D. (2005) STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane, Nature 437, 902–905.
Lee, K. P., Yuan, J. P., Hong, J. H., So, I., Worley, P. F., and Muallem, S. (2009) An endoplasmic reticulum/plasma membrane junction: STIM1/Orai1/TRPCs, FEBS Lett 584, 2022–2027.
Chiu, W. T., Tang, M. J., Jao, H. C., and Shen, M. R. (2008) Soft substrate up-regulates the interaction of STIM1 with store-operated Ca2+ channels that lead to normal epithelial cell apoptosis, Mol Biol Cell 19, 2220–2230.
Guo, R. W., Wang, H., Gao, P., Li, M. Q., Zeng, C. Y., Yu, Y., Chen, J. F., Song, M. B., Shi, Y. K., and Huang, L. (2009) An essential role for stromal interaction molecule 1 in neointima formation following arterial injury, Cardiovasc Res 81, 660–668.
Feske, S., Gwack, Y., Prakriya, M., Srikanth, S., Puppel, S. H., Tanasa, B., Hogan, P. G., Lewis, R. S., Daly, M., and Rao, A. (2006) A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function, Nature 441, 179–185.
Picard, C., McCarl, C. A., Papolos, A., Khalil, S., Luthy, K., Hivroz, C., LeDeist, F., Rieux-Laucat, F., Rechavi, G., Rao, A., Fischer, A., and Feske, S. (2009) STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity, N Engl J Med 360, 1971–1980.
Feske, S., Prakriya, M., Rao, A., and Lewis, R. S. (2005) A severe defect in CRAC Ca2+ channel activation and altered K + channel gating in T cells from immunodeficient patients, J Exp Med 202, 651–662.
Bergmeier, W., Oh-Hora, M., McCarl, C. A., Roden, R. C., Bray, P. F., and Feske, S. (2009) R93W mutation in Orai1 causes impaired calcium influx in platelets, Blood 113, 675–678.
Takahashi, Y., Watanabe, H., Murakami, M., Ono, K., Munehisa, Y., Koyama, T., Nobori, K., Iijima, T., and Ito, H. (2007) Functional role of stromal interaction molecule 1 (STIM1) in vascular smooth muscle cells, Biochem Biophys Res Commun 361, 934–940.
Author information
Authors and Affiliations
Corresponding authors
Additional information
DR. JENNIFER A. TIMMONS (University of Maryland, Baltimore, MD)
DISCUSSION
DR. TEREZ SHEA Donohue (University of Maryland, Baltimore, MD): Thank you for a wonderful talk. My question is more related to the STIM1 expression. You are using a cell line where you are overexpressing STIM1. And so I wanted to know if there's any information on the regulation of STIM1, and if so, under what conditions you might anticipate where it might be used as a proposed therapeutic target in terms of looking at STIM1 expression?
DR. JENNIFER A. TIMMONS: What we know is that STIM1 acts as a calcium sensor and also plays a role in cell growth and proliferation. We don't know all aspects of the pathway in terms of what stimulates this activation and its downstream effectors and target proteins. Since STIM1 is important for activation of SOC channels, which are important in mimicking cellular physiology and targeting diseases such as IBD (Crohn's disease and ulcerative colitis), as well as use in immune regulation and potential cancer therapies.
DR. RICHARD WALDRON (UCLA, Los Angeles, CA): I think this is extremely fascinating. I could probably come up with dozens of questions. But I think the ones that are really salient would be first, it's fascinating that you have obviously elevated calcium in the STIM1 overexpressing IEC-6 cells. You showed that sort of a basal, constitutive influx, if you will. And then you see a much more massive—you are actually doing like an overshoot response where you have no calcium in the extracellular medium while you are depleting the stores, and then you add it back after about two minutes and that gives a very pronounced response.
So I'm wondering—but at the same time you have more apoptosis in those cells. So it seems that calcium is better for growth and it's also better for apoptosis.
So with that said, my first question is, do you see calcium responses with TNF and cycloheximide? Is there correlation between your specific treatment and the changes in calcium that presumably result in greater apoptosis?
DR. JENNIFER TIMMONS: We did our experiments in calcium free medium in the presence of TNF-alpha and cycloheximide. What we know from these experiments and previous ones from our group is that TNF-alpha and cycloheximide under calcium free conditions sensitizes IEC cells to apoptosis when compared to cells grown in normal medium. This was partially reversed in STIM1 overexpression cells.
DR. RICHARD WALDRON: Just another brief question. I guess it would come down to the measurement of apoptosis. I know in that cell line it's kind of a pronounced shedding response where a lot of cells get released from the mono layer. And if you have sort of overgrowth to begin with, there might be a much faster—many more cells. I assume you're counting the cells that come off the mono layer as part of the apoptotic cells.
JENNIFER A. TIMMONS: Yes. We do total cell counts. Because the STIM1 cells grow faster, I have to plate them at a lower density so that the densities are equal between the parent cell line and the STIM1 cells, so that I don't end up with overgrowth confounding my data.
DR. RICHARD WALDRON: So they start out equal numbers, and then you count the ones that come off, or the ones that you assess as apoptotic?
JENNIFER A. TIMMONS: In terms of the protein, it's the total. So it is the ones that come off as well as whatever is scraped from the plate.
DR. RICHARD WALDRON (UCLA): So to do that you have to spin the medium and collect it. Okay. Thank you very much.
DR. JEFF MATTHEWS (University of Chicago): That was a beautiful presentation. If you visualize STIM1 in your overexpressing cells, does it stay in the ER, or is it now being expressed in plasma membrane constitutively?
DR. JENNIFER A. TIMMONS: We haven't personally visualized it. But what I do know is that it kind of plays both roles. In some situations, it translocates to the plasma membrane and interacts with the calcium channels. There's also a circumstance where it seems like other people's studies have shown that the endoplasmic reticulum comes closer to the plasma membrane and STIM1 forms a complex, or groups together, within the ER to possibly interact with calcium channels that way.
DR. JEFF MATTHEWS: You might just want to check it specifically in the overexpressing cells. It's important to know if the distribution stays normal because it may change and that may explain some of the results.
DR. JENNIFER A. TIMMONS: Yes. We haven't looked at that yet.
This research was supported by a Merit Review Grants from the Department of Veterans Affairs (JNR, DJT, JYW) and by grants from National Institute of Diabetes and Digestive and Kidney Diseases (JYW) including a T32 DK067872 (JAT) Research Training in Gastroenterology.
This research was presented at Digestive Diseases Week (DDW) on May 30 and June 2, 2009 in Chicago, IL.
Rights and permissions
About this article
Cite this article
Timmons, J.A., Rao, J.N., Turner, D.J. et al. Induced Expression of STIM1 Sensitizes Intestinal Epithelial Cells to Apoptosis by Modulating Store-Operated Ca2+ Influx. J Gastrointest Surg 16, 1397–1405 (2012). https://doi.org/10.1007/s11605-012-1876-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-012-1876-8