Abstract
Introduction
Butyrate is a bacterial fermentation product that produces its beneficial effects on colon through GPR109A, a butyrate receptor, and SLC5A8, a butyrate transporter. In this study, we compared the expression of GPR109A and SLC5A8 between conventional mice and germ-free mice to test the hypothesis that the expression of these two proteins will be decreased in germ-free mice compared to conventional mice because of the absence of bacterial fermentation products and that colonization of germ-free mouse colon with conventional bacteria will reverse these changes.
Methods
RNA was prepared from the ileum and colon of conventional mice and germ-free mice and used for RT-PCR to determine mRNA levels. Tissue sections were used for immunohistochemical analysis to monitor the expression of GPR109A and SLC5A8 at the protein level. cDNA microarray was used to determine the differential expression of the genes in the colon between conventional mice and germ-free mice.
Results
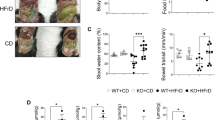
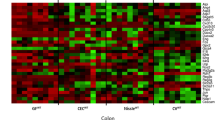
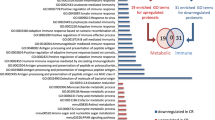
In conventional mice with normal bacterial colonization of the intestinal tract, GPR109A and SLC5A8 are expressed on the apical membrane of epithelial cells lining the ileum and colon. In germ-free mice, the expression of GPR109A and SLC5A8 is reduced markedly in the ileum and colon. The expression returns to normal levels when the intestinal tract of germ-free mice is colonized with bacteria. The expression of the Na+-coupled glucose transporter, SGLT1, follows a similar pattern. Microarray analysis identifies ∼700 genes whose expression is altered more than twofold in germ-free mice compared to conventional mice. Among these genes are the chloride/bicarbonate exchanger SLC26A3 and the water channel aquaporin 4. The expression of SLC26A3 and AQP4 in ileum and colon is reduced in germ-free mice, but the levels return to normal upon bacterial colonization.
Conclusion
Gut bacteria play an active role in the control of gene expression in the host intestinal tract, promoting the expression of the genes that are obligatory for the biological actions of the bacterial fermentation product butyrate and also the genes that are related to electrolyte and water absorption.




Similar content being viewed by others
References
Hooper LVE, Midtvedt T, Gordon JI. How host–microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr 2002;22:283–307.
Backhed F, Ley R, Sonnenburg JL, Peterson DA, Gordon JI. Host–bacterial mutualism in the human intestine. Science 2005;307:1915–1920.
Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol 1977;31:107–133.
Peterson DA, Frank DN, Pace NR, Gordon JI. Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases. Cell Host Microbe 2008;3:417–427.
Mitsuyama K, Sata M. Gut microflora: a new target for therapeutic approaches in inflammatory bowel disease. Expert Opin Ther Targets 2008;12:301–312.
Knight P, Campbell BJ, Rhodes JM. Host–bacteria interaction in inflammatory bowel disease. Br Med Bul 2008;88:95–113.
Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 2001;81:1031–1064.
Mortensen PB, Clausen MR. Short-chain fatty acids in the human colon: relation to gastrointestinal health and disease. Scand J Gastroenterol 1996;216:132–148.
Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol 2006;40:235–243.
Sellin JH. SCFAs: the enigma of weak electrolyte transport in the colon. News Physiol Sci 1999;14:58–64.
Roy CC, Kien CL, Bouthillier L, Levy E. Short-chain fatty acids: ready for prime time? Nutr Clin Pract 2006;21:351–366.
Bordonaro M, Lazarova DL, Sartorelli AC. Butyrate and Wnt signaling. Cell Cycle 2008;7:1178–1183.
Miyauchi S, Gopal E, Fei YJ , Ganapathy, V. Functional identification of SLC5A8, a tumor suppressor down-regulated in colon cancer, as a Na+-coupled transporter for short-chain fatty acids. J Biol Chem 2004;279:13293–13296.
Coady MJ, Chang MH, Charron FM, Plata C, Wallendorff B, Sah J et al. The human tumour suppressor gene SLC5A8 expresses a Na+-monocarboxylate cotransporter. J Physiol 2004;557:719–731.
Gopal E, Miyauchi S, Martin PM, Ananth S, Roon P, Smith SB, Ganapathy V. Transport of nicotinate and structurally related compounds by human SMCT1 (SLC5A8) and its relevance to drug transport in the mammalian intestinal tract. Pharm Res 2007;24:575–584.
Takebe K, Nio J, Morimatsu M, Karaki S, Kuwahara A, Kato I et al. Histochemical demonstration of a Na+-coupled transporter for short-chain fatty acids (slc5a8) in the intestine and kidney of the mouse. Biomed Res 2005;26:213–221.
Thangaraju M, Cresci GA, Itagaki S, Mellinger JD, Browning DD, Berger FG, Prasad PD, Ganapathy V. Sodium-coupled transport of the short chain fatty acid butyrate by SLC5A8 and its relevance to colon cancer. J Gastrointest Surg 2008;12:1773–1781.
Ganapathy V, Gopal E, Miyauchi S, Prasad PD. Biological functions of SLC5A8, a candidate tumour suppressor. Biochem Soc Trans 2005;33:237–240.
Gupta N, Martin PM, Prasad PD, Ganapathy V. SLC5A8 (SMCT1)-mediated transport of butyrate forms the basis for the tumor suppressive function of the transporter. Life Sci 2006;78:2419–2425.
Ganapathy V, Thangaraju M, Gopal E, Itagaki S, Miyauchi S, Prasad, PD. Sodium-coupled monocarboxylate transporters in normal tissues and in cancer. AAPS J 2008;10:193–199.
Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: Relevance to Warburg hypothesis and beyond. Pharmacol Ther 2009;121:29–40.
Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, Browning DD et al. GPR109A is a G-protein coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res 2009;69:2826–2832.
Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host–microbial relationships in the intestine. Science 2001;291:881–884.
Hooper LV, Stappenbeck TS, Hong CV, Gordon JI. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol 2003;4:269–273.
Gopal E, Umapathy NS, Martin PM, Gnana-Prakasam JP, Becker H, Wagner CA et al. Cloning and functional characterization of human SMCT2 (SLC5A12) and expression pattern of the transporter in kidney. Biochim Biophys Acta 2007;1768:2690–2697.
Martin PM, Ananth S, Cresci GA, Roon P, Smith SB, Ganapathy V. Expression and localization of GPR109A (PUMA-G/HM74A) mRNA and protein in mammalian retinal pigment epithelium. Mol Vis 2009;15:362–372.
Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 1998;95:14863–14868.
Liu K, Caldwell SA, Abrams SI. Immune selection and emergence of aggressive tumor variants as negative consequences of Fas-mediated cytotoxicity and altered IFN-gamma-regulated gene expression. Cancer Res 2005;65:4376–4388.
Wise A, Foord SM, Fraser JN, Barnes AA, Elshourbagy N, Eilert M et al. Molecular identification of high and low affinity receptors for nicotinic acid. J Biol Chem 2003;278:9869–9874.
Tunaru S, Kero J, Schaub A, Wufka C, Blaukat A, Pfeffer K et al. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat Med 2003;9:352–355.
Taggart AKP, Kero J, Gan X, Cai TQ, Cheng K et al. (D)-β-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem 2005;280:26649–26652.
Ganguly NK, Kaur T. Mechanism of action of cholera toxin & other toxins. Ind J Med Res 1996;104:28–37.
Field M, Semrad CE. Toxigenic diarrheas, congenital diarrheas, and cystic fibrosis: Disorders of intestinal ion transport. Annu Rev Physiol 1993;55:631–655.
Field M. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest 2003;111:931–943.
Acknowledgments
We thank Krishnan Dhandapani, PhD (Department of Neurosurgery, Medical College of Georgia) for the monoclonal antibody specific for AQP4. This work was supported by the Scientist Training Program from the Medical College of College of Georgia Research Institute, Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
Discussant
DR. ROBERT MARTINDALE (Portland, OR): Thank you, Gail, for this very interesting work. This shows us that we truly are mutualistic with our bacteria as this paper and many others at this meeting have shown us.
There are several things you have very nicely shown through microarray and immunohistofluorescence data.
The question I have for you today is regarding the mechanism. Is it the bacteria themselves or some product of the bacteria? Can you speculate on the mechanism?
Discussant
GAIL CRESCI: Thank you for your very interesting question. We were interested in that question as well. We’ve done some preliminary work, and I don’t have the data to show here, but because we saw down-regulation of the two genes that we are studying, SLC5A8 and GPR109A, and that we have previously shown that they are silenced in colon cancer by DNA methylation, we sought out to see if this might be the possible cause with germ-free animals.
Our preliminary work has actually shown that, in fact, for the silencing of these two genes, SLC5A8 and GPR109A, DNA methylation is definitely involved and DNMT1 seems to be the main isoform involved.
We also know butyrate alters gene expression as shown in other people’s work. One may predict there to be an absence of butyrate in a germ-free mouse intestine as the production of butyrate results from bacterial fermentation of undigested polysaccharides. Thus with the absence of this molecule, gene expression may be altered.
I have attended many lectures here at this conference and have read many papers that lead to the thought that perhaps the bacteria itself may be influencing gene expression by secreting proteins or altering the lumen pH. So, without the presence of the gut microbiota, these proteins and other alterations would not exist. That is future work for us. Thank you.
Discussant
DR. ROBERT MARTINDALE (Portland, OR): Do you think having two mechanisms, including the receptor you’ve shown, as well as a transporter, shows the importance of butyrate? Thus, if one is knocked out, you still have another alternate way for butyrate to elicit its biologic effects on the cell?
Discussant
GAIL CRESCI: Yes. I think that’s very important. We are very excited to see that both have a relationship with butyrate. We actually now have knockout mice for GPR109A as well as SLC5A8. So we are, in fact, going to start some studies looking at the potential mechanism there.
Discussant
DR. TEREZ SHEA-DONOHUE (Baltimore): I was interested that you have SGLT1 expression in the colon and one doesn’t normally think of the colon as having a nutrient transporter like that. Can you comment on what you think the role of the transporter is there?
Closing discussant
GAIL CRESCI: We were surprised by that as well. Just speculating as I really wouldn’t expect to find glucose in the colon. I’ve been to other presentations this weekend showing that some of these different micobiota rely on different nutrient sources. So perhaps the presence of glucose in the colon is for that or to help with sodium and water absorption as well.
Rights and permissions
About this article
Cite this article
Cresci, G.A., Thangaraju, M., Mellinger, J.D. et al. Colonic Gene Expression in Conventional and Germ-Free Mice with a Focus on the Butyrate Receptor GPR109A and the Butyrate Transporter SLC5A8. J Gastrointest Surg 14, 449–461 (2010). https://doi.org/10.1007/s11605-009-1045-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-009-1045-x