Abstract
Introduction
The presentation and outcome of patients with acinar cell carcinoma (ACC) of the pancreas compared to the more common ductal cell adenocarcinoma (DCA) may be distinct. This study combines the experience with ACC from multiple academic institutions to better understand its natural history and outcomes.
Methods
This study is a multi-institutional retrospective review of patients with ACC.
Results
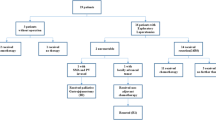
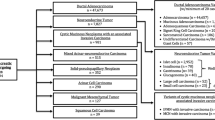
Between 1988 and 2008, 17 patients were identified with pathologically confirmed ACC. Median age at presentation was 59 years. Common presenting symptoms were abdominal pain (60%), back pain (50%), and weight loss (45%). Fifteen patients underwent 16 operations: pancreaticoduodenectomy (nine), distal pancreatectomy (four), and exploratory laparotomy (three). Mean tumor size was 5.3 cm. American Joint Commission on Cancer tumor stages were stage I (two), stage II (eight), stage III (four), and stage IV (three). Overall, 1- and 5-year survival rates were 88% and 50%, respectively. In resected cases (13), 1- and 5-year survival rates were 92% and 53%, respectively. Median survival in resected cases was 61 months. This is in contrast to 1,608 patients with ductal cell adenocarcinoma who underwent resection identified from recent literature reports where the average median survival was only 24 months. There was no discernable difference in the outcomes of patients with ACC between United States and Germany patients.
Conclusion
Acinar cell carcinoma of the pancreas is rare and appears to have a presentation and outcome distinct from the more common pancreatic DCA. Based upon these data, the outcome of patients with ACC is superior to that of DCA.



Similar content being viewed by others
References
Williams JA. Regulation of pancreatic acinar cell function. Curr Opin Gastroenterol 2006;22:498–504.
Berner. Subkutane fettgewebsnekose. Virchow Arch Pathol Anat 1908;193:510–518.
Robertson JC, Eeles GH. Syndrome associated with pancreatic acinar cell carcinoma. Br Med J 1970;2:708–709.
Kitagami H, Kondo S, Hirano S, Kawakami H, Egawa S, Tanaka M. Acinar cell carcinoma of the pancreas: clinical analysis of 115 patients from Pancreatic Cancer Registry of Japan Pancreas Society. Pancreas 2007;35:42–46.
Mulkeen AL, Yoo PS, Cha C. Less common neoplasms of the pancreas. World J Gastroenterol 2006;12:3180–3185.
Chen J, Baithun SI. Morphological study of 391 cases of exocrine pancreatic tumours with special reference to the classification of exocrine pancreatic carcinoma. J Pathol 1985;146:17–29.
Hartman GG, Ni H, Pickleman J. Acinar cell carcinoma of the pancreas. Arch Pathol Lab Med 2001;125:1127–1128.
Ordonez NG. Pancreatic acinar cell carcinoma. Adv Anat Pathol 2001;8:144–159.
Ordonez NG, Mackay B. Acinar cell carcinoma of the pancreas. Ultrastruct Pathol 2000;24:227–241.
Klimstra DS, Heffess CS, Oertel JE, Rosai J. Acinar cell carcinoma of the pancreas. A clinicopathologic study of 28 cases. Am J Surg Pathol 1992;16:815–837.
Holen KD, Klimstra DS, Hummer A, Gonen M, Conlon D, Brennan M, Saltz LB. Clinical characteristics and outcomes from an institutional series of acinar cell carcinoma of the pancreas and related tumors. J Clin Oncol 2002;20:4673–4678.
Webb JN. Acinar cell neoplasms of the exocrine pancreas. J Clin Pathol 1977;30:103–112.
Cubilla AL, Fitzgerald PJ. Classification of pancreatic cancer (nonendocrine). Mayo Clin Proc 1979;54:449–458.
Khalili M, Wax BN, Reed WP, Schuss A, Drexler S, Weston SR, Katz DS. Radiology–pathology conference. Acinar cell carcinoma of the pancreas. Clin Imaging 2006;30:343–346.
Seth AK, Argani P, Campbell KA, Cameron JL, Pawlik TM, Schulick RD, Choti MA, Wolfgang CL. Acinar cell carcinoma of the pancreas: an institutional series of resected patients and review of the current literature. J Gastrointest Surg 2008;12:1061–1067.
Nitecki SS, Sarr MG, Colby TV, van Heerden JA. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg 1995;221:59–66.
Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, Sauter PK, Coleman J, Hruban RH, Lillemoe KD. Resected adenocarcinoma of the pancreas—616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg 2000;4:567–579.
Breslin TM, Hess KR, Harbison DB, Jean ME, Cleary KR, Dackiw AP, Wolff RA, Abbruzzese JL, Janjan NA, Crane CH, Vauthey JN, Lee JE, Pisters PW, Evans DB. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: treatment variables and survival duration. Ann Surg Oncol 2001;8:123–132.
Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, Gutberlet K, Kettner E, Schmalenberg H, Weigang-Koehler K, Bechstein WO, Niedergethmann M, Schmidt-Wolf I, Roll L, Doerken B, Riess H. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267–277.
Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PW, Vauthey JN, Wang H, Cleary KR, Staerkel GA, Charnsangavej C, Lano EA, Ho L, Lenzi R, Abbruzzeese JL, Wolff RA. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:3496–3502.
Varadhachary GR, Wolff RA, Crane CH, Sun CC, Lee JE, Pisters PW, Vauthey JN, Abdalla E, Wang H, Staerkel GA, Lee JH, Ross WA, Tamm EP, Bhosale PR, Drishnan S, Das P, Ho L, Xiong H, Abbruzzese JL, Evans DB. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:3487–3495.
Turrini O, Viret F, Moureau-Zabotto L. Ten years experience with neoadjuvant radiochemotherapy for patients with resectable adenocarcinoma of the pancreatic head. Oncology 2009 (in press).
Katz MH, Wang H, Fleming JB, Sun CC, Hwang RF, Wolff RA, Varadhachary G, Abbruzzese JL, Crane CH, Krishnan S, Vauthey JN, Abdalla EK, Lee JE, Pisters PW, Evans DB. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol 2009;15:836–847.
Wisnoski NC, Townsend CM Jr., Nealon WH, Freeman JL, Riall TS. 672 patients with acinar cell carcinoma of the pancreas: a population-based comparison to pancreatic adenocarcinoma. Surgery 2008;144:141–148.
Schmidt CM, Matos JM, Bentrem DJ, Talamonti MS, Lillemoe KD, Bilimoria KY. Acinar cell carcinoma of the pancreas in the United States: prognostic factors and comparison to ductal adenocarcinoma. J Gastrointest Surg 2008;12:2078–2086.
Ohike N, Kosmahl M, Kloppel G. Mixed acinar–endocrine carcinoma of the pancreas. A clinicopathological study and comparison with acinar–cell carcinoma. Virchows Arch 2004;445:231–235.
Morohoshi T, Kanda M, Horie A, Chott A, Dreyer T, Kloppel G, Heitz PU. Immunocytochemical markers of uncommon pancreatic tumors. Acinar cell carcinoma, pancreatoblastoma, and solid cystic (papillary-cystic) tumor. Cancer 1987;59:739–747.
Klimstra DS, Rosai J, Heffess CS. Mixed acinar–endocrine carcinomas of the pancreas. Am J Surg Pathol 1994;18:765–778.
Basturk O, Zamboni G, Klimstra DS, Capelli P, Andea A, Kamel NS, Adsay NV. Intraductal and papillary variants of acinar cell carcinomas: a new addition to the challenging differential diagnosis of intraductal neoplasms. Am J Surg Pathol 2007;31:363–370.
Hashimoto M, Matsuda M, Watanabe G, Mori M, Motoi N, Nagai K, Ishibashi M. Acinar cell carcinoma of the pancreas with intraductal growth: report of a case. Pancreas 2003;26:306–308.
Svrcek M, Lesurtel M, Lewin M, Afchain P, Fabre M, Scoazec JY, Parc R, Flejou JF. Acinar cell carcinoma of the pancreas with predominant intraductal growth: report of a case. Gastroenterol Clin Biol 2007;31:543–546.
Fabre A, Sauvanet A, Flejou JF, Belghiti J, Palazzo L, Ruszniewski P, Degott C, Terris B. Intraductal acinar cell carcinoma of the pancreas. Virchows Arch 2001;438:312–315.
Sawey ET, Johnson JA, Crawford HC. Matrix metalloproteinase 7 controls pancreatic acinar cell transdifferentiation by activating the Notch signaling pathway. Proc Natl Acad Sci USA 2007;104:19327–19332.
Means AL, Meszoely IM, Suzuki K, Miyamoto Y, Rustgi AK, Coffey RJ, Wright CV, Stoffers DA, Leach SD. Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development 2005;132:3767–3776.
Schmid RM. Acinar-to-ductal metaplasia in pancreatic cancer development. J Clin Invest 2002;109:1403–1404.
Zhu L, Tran T, Rukstalis JM, Sun P, Damsz B, Konieczny SF. Inhibition of Mist1 homodimer formation induces pancreatic acinar-to-ductal metaplasia. Mol Cell Biol 2004;24:2673–2681.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jesus M. Matos and C. Max Schmidt contributed equally to this work.
Rights and permissions
About this article
Cite this article
Matos, J.M., Schmidt, C.M., Turrini, O. et al. Pancreatic Acinar Cell Carcinoma: A Multi-institutional Study. J Gastrointest Surg 13, 1495–1502 (2009). https://doi.org/10.1007/s11605-009-0938-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-009-0938-z