Abstract
Introduction
Pancreatic cancer (PC) is the fourth leading cause of cancer death in the United States. This study characterizes one of the largest national registries of familial PC (FPC) and sporadic PC (SPC), focusing on demographics, clinical factors, self-reported environmental and occupational lifetime exposures, and survival status.
Background
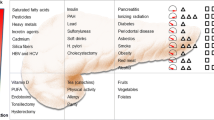
Reported risk factors for PC include advancing age, a family history of PC, high-risk inherited syndromes, cigarette, cigar, and pipe smoking, exposure to occupational and environmental carcinogens, African-American race, high fat/high cholesterol diet, obesity, chronic pancreatitis, and diabetes mellitus.
Patients and Methods
This retrospective cross-sectional, case-only analysis includes cases of FPC (n = 569) and SPC (n = 689) from the Johns Hopkins National Familial Pancreas Tumor Registry (NFPTR) enrolled between 1994 and 2005.
Results
FPC smokers with environmental tobacco smoke (ETS) exposure were diagnosed at a significantly younger mean age (63.7 years) as compared to FPC non-smokers without ETS exposure (66.6 years; p = 0.05). Non-smoker ETS-exposed cases were diagnosed with PC at a significantly younger mean age (64.0 years) compared to non-smoker non-ETS-exposed cases (66.5 years) (p < 0.0004). The mean age at diagnosis for Ashkenazi Jewish SPC subjects was significantly younger (by 2.1 years) than Ashkenazi Jewish FPC cases (p = 0.05). In addition, Ashkenazi Jewish FPC subjects who smoked were diagnosed 5.9 years earlier than Ashkenazi Jewish FPC non-smokers (p = 0.05). The median length of survival for unresected FPC cases was significantly shorter (168 days) as compared to unresected SPC cases (200 days) (p = 0.04). Survival was improved in resected cases, 713 days for FPC cases and 727 days for SPC cases, but was not significantly different between the groups (p = 0.4). Mild to moderate multiplicative interaction was found between a family history of PC and exposure to asbestos, environmental radon, and environmental tobacco smoke (ETS), as evidenced by odds ratios >1.0.
Conclusions
These are the first data to show that occupational and environmental exposures may act synergistically with inherited or acquired genetic polymorphisms, resulting in earlier occurrence of PC. Exposure to cigarette smoking and ETS exposure in non-smokers when younger than 21 years of age are associated with a younger mean age of diagnosis in FPC and SPC cases and Ashkenazi Jewish smokers, when compared to non-exposed cases. Risk prediction models which take into account environmental exposures as well as family history may more accurately predict the risk of PC. High-risk individuals will likely benefit from early identification of pre-malignant lesions and molecular profiling, as methods of early detection, prevention, and personalized therapy.
Similar content being viewed by others
References
American Cancer Society. Cancer facts and figures. Atlanta: American Cancer Society, 2008.
Hruban R, Petersen G, Ha P, Kern S. Genetics of pancreatic cancer. Surg Oncol Clin N Am 1998;7(1):1–23.
Arnold M, Goggins M. BRCA2 and predisposition to pancreatic and other Cancers. Expert Reviews in Molecular Medicine:http://www-ermm.cbcu.cam.ac.uk Accession information (01)00309-Xh.htm(shortcode:txt001mgb); 14 May 2001.
Gold E, Goldin S. Epidemiology of and risk factors for pancreatic cancer. Surg Oncol Clin N Am 1998;7(1):67–91.
Lowenfels A, Maisonneuve E. Epidemiologic and etiologic factors of pancreatic cancer. Hematol Oncol Clin North Am 2002;16(1):1–16. doi:10.1016/S0889-8588(01)00003-X.
Lowenfels A, Maisonneuve E. Epidemiology and prevention of pancreatic cancer. Jpn J Clin Oncol 2004;34(5):238–244. doi:10.1093/jjco/hyh045.
Iodice S, Gandini S, Maisonneuve P, Lowenfels A. Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch Surg 2008;393:535–545. doi:10.1007/s00423-007-0266-2.
Yeo T, Hruban R, Leach S, Wilentz R, Sohn T, Kern S, Iacobuzio-Donahue C, Maitra A, Goggins M, Canto M, Abrams R, Laheru D, Jaffee E, Hidalgo M, Yeo CJ. Pancreatic cancer. Curr Probl Cancer 2002;26(4):165–276. doi:10.1067/mcn.2002.129579.
Silverman D, Dunn J, Hoover R, Schiffman M, Lillemoe K, Schoenberg J, Brown L, Greenberg R, Hayes R, Swanson M, Wacholder S, Schwartz A, Liff J, Pottern L. Cigarette smoking and pancreas cancer: a case–control study based on direct interviews. J Natl Cancer Inst 1994;86(20):1510–1516. doi:10.1093/jnci/86.20.1510.
Howe G, Jain M, Burch J, Miller A. Cigarette smoking and cancer of the pancreas: evidence from a population-based case–control study in Toronto, Canada. Int J Cancer 1991;47:323–328. doi:10.1002/ijc.2910470302.
Ishii K, Nakamura K, Okzzaki H et al. (in Japanese). Nippon Rinsho, 26, 1839–1842. (quoted in Ahlgren, Ahlgren, J. (1996). Epidemiology and risk factors in pancreatic cancer. Semin Oncol 1968;23(2):241–250.
Husgafvel-Pursiainen K. Genotoxicity of environmental tobacco smoke: a review. Mutat Res 2004;567:427–445. doi:10.1016/j.mrrev.2004.06.004.
Kasim K, Levallois P, Abdous B, Auger P, Johnson K, The Canadian Cancer Registries Epidemiology Research Group. Environmental tobacco smoke and risk of adult leukemia. Epidemiology 2005;16(5):672–680. doi:10.1097/01.ede.0000173039.79207.80.
National Cancer Institute. (March 7, 2000). Cancer Facts. Accessed at: http://cis.nci.nih.gov/fact/3_65.htm. National Center for Health Statistics. Health, United States, 2000 with Urban and Rural Chartbook. Hyattsville, MD: Public Health Service; 2001.
International Agency for Research on Cancer (IARC). Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risk Chem Hum 2004;83:1–1452.
Villeneuve P, Johnson K, Hanley A. Environmental tobacco smoke and the risk of pancreatic cancer: findings from a Canadian population-based case–control study. Can J Public Health 2004;95(1):32–37.
Falk R, Pickle L, Fontham E, Correa P, Morse A, Chen V, Fraumeni J. Occupation and pancreatic cancer risk in Louisiana. Am J Ind Med 1990;18:565–576.
Rotimi C, Austin H, Delzell E, Day C, Macaluso M, Honda Y. Retrospective follow-up study of foundry and engine plant workers. Am J Ind Med 1993;24:485–498.
Ojajarvi I, Partanen T, Ahlbom A, Biffetta P, Hakulinen T, Jourenkova N, Kauppinen T, Kogevinas M, Porta M, Vainio H, Weiderpass E, Wesseling C. Occupational exposures and pancreatic cancer: a meta-analysis. Occup Environ Med 2000;57(5):316–324.
Bu-Tian J, Silverman D, Stewart P, Blair A, Swanson G, Bartis D, Greenberg R, Hayes R, Brown L, Lillemoe K, Schoenberg J, Pottern L, Schwartz, Hoover R. Occupational exposures to pesticides and pancreatic cancer. Am J Ind Med 2001;39:92–99.
Krush A, Giardiello F. Development of a genetics registry: Hereditary intestinal polyposis and hereditary colon cancer registry at The Johns Hopkins Hospital, 1973–1988. In Herrera L, ed. Familial adenomatous polyposis. New York: Liss, 1990, pp 43–59.
Gauderman J. Quanto®. Version 1.0. Accessed: http://hydra.usc.edu/gxe Stata® Version 7.0 (2002). Stata Corporation, College Station, Texas. http://www.stata.com, 2002.
U.S. Department of Labor, Bureau of Labor Statistics. 2000 Standard Occupational Classification User Guide (on-line). Accessed 3/4/06. at: http://www.bls.gov/soc/socguide.htm.
Hecht S. Cigarette smoking and lung cancer: chemical mechanisms and approaches to prevention. Lancet Oncol 2002;1:461–469.
Diethelm P, Rielle J, McKee M. The whole truth and nothing but the truth? The research that Philip Morris did not want you to see. Lancet 2005;366:86–92.
Mohtashamipur E, Mohtashamipur A, Germann P, Ernst H, Norpoth K, Mohr U. Comparative carcinogenicity of cigarette mainstream and sidestream smoke condensates on the mouse skin. J Cancer Res Clin Oncol 1990;116:604–608.
McWilliams R, Bamlet W, Cunningham J, Goode E, de Andrade M, Boardman L, Petersen G. Polymorphisms in DNA repair genes, smoking, and pancreatic adenocarcinoma risk. Cancer Res 2008;68:4928–4935.
Lee PN. Environmental tobacco smoke and cancer of sites other than the lung in adult non-smokers. Food Chem Toxicol 2002;40(6):747–766.
Brownson R, Figgs L, Caisley L. Epidemiology of environmental smoke and lung cancer in nonsmoking women. Oncogene 2002;21:7341–7348. 30.
Wang W, Chen S, Brune K, Hruban R, Parmigiani G, Klein A. PancPRO: risk assessment for individuals with a family history of pancreatic cancer. J Clin Oncol 2007;25:1417–1422.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yeo, T.P., Hruban, R.H., Brody, J. et al. Assessment of “Gene–Environment” Interaction in Cases of Familial and Sporadic Pancreatic Cancer. J Gastrointest Surg 13, 1487–1494 (2009). https://doi.org/10.1007/s11605-009-0923-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-009-0923-6