Abstract
Aim
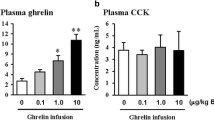
Ghrelin, the most important modulator of endocrine and exocrine pancreatic functions, has a role in the development of islets of Langerhans during embryogenesis. The aim of this study was to evaluate the effects of ghrelin on pancreatic regeneration in rats with 90% pancreatectomy.
Materials and Methods
Two- to 3-week-old Wistar rats were used in the study. After anesthesia, 90% pancreatectomy was performed. In the ghrelin group, 90% pancreatectomy was performed. Ten nanomoles per kilogram per day of ghrelin was administered intraperitoneally from the first postoperative day. In the antagonist group, 90% pancreatectomy was performed. From the first postoperative day, rats received the ghrelin receptor antagonists and substance P intraperitoneally at 1 μmol/kg. In the control group, 90% pancreatectomy was performed, and intraperitoneal saline was administered. The sham group did not receive pancreatectomy. Eight rats from each group were randomly selected and sacrificed on the second, third, and 30th days.
Results
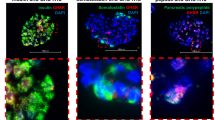
Blood glucose levels in pacreatectomized rats were significantly higher than in rats in the sham group. The number of beta islet cells, serum insulin levels, and PDX-1 and cytokeratin staining scores decreased in rats with pancreatectomy when compared to the sham-group rats. In the ghrelin-receiving rats, blood glucose levels tended to decrease from the 15th postoperative day. Ghrelin treatment increased insulin levels, insulin-positive islet cell number, and 5-bromo-2-deoxyuridine and PDX-1 staining, whereas ghrelin antagonist administration resulted in significant decreases in these parameters. Ghrelin treatment significantly improved glucose tolerance test results.
Conclusion
Exogenous ghrelin administration decreased blood glucose levels after 90% pancreatectomy by increasing islet cell numbers and enhancing endocrine and exocrine regeneration.









Similar content being viewed by others
References
Terazono K, Uchiyama Y, Ide M, Watanabe T, Yonekura H, Yamamoto H, Okamoto H. Expression of reg protein in rat regenerating islets and its co-localization with insulin in the beta cell secretory granules. Diabetologia 1990;33:250–252. doi:10.1007/BF00404804.
Brockenbrough JS, Weir GC, Bonner-Weir S. Discordance of exocrine and endocrine growth after 90% pancreatectomy in rats. Diabetes 1988;37:232–236. doi:10.2337/diabetes.37.2.232.
Rafaeloff R, Pittenger GL, Barlow SW, Qin XF, Yan B, Rosenberg L, Duguid WP, Vinik AI. Cloning and sequencing of the pancreatic islet neogenesis-associated protein (INGAP) gene and its expression in islet neogenesis in hamsters. J Clin Invest 1997;99:2100–2109. doi:10.1172/JCI119383.
Gu D, Sarvetnick N. Epithelial cell proliferation and islet neogenesis in IFN-g transgenic mice. Development 1993;118:33–46.
Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic β cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004;429:41–46. doi:10.1038/nature02520.
List JF, Habener JF. Glucagon-like peptide 1 agonists and the development and growth of pancreatic beta cells. Am J Physiol Endocrinol Metab 2004;286:E875–E881. doi:10.1152/ajpendo.00007.2004.
Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both b-cell replication and neogenesis, resulting in increased β-cell mass and improved glucose tolerance in diabetic rats. Diabetes 1999;48:2270–2276. doi:10.2337/diabetes.48.12.2270.
Pospislik JA, Martin J, Doty T, Ehses JA, Pamir N, Lynn FC, Piteau S, Demuth HU, McIntosh CH, Pederson RA. Dipeptidyl peptidase IV inhibitor treatment stimulates β-cell survival and islet neogenesis in streptozotocin-induced diabetic rats. Diabetes 2003;52:741–750. doi:10.2337/diabetes.52.3.741.
Massague J, Chen YG. Controlling TGF-beta signaling. Genes Dev 2000;14:627–644.
Hebrok M, Kim SK, Melton DA. Notochord repression of endodermal sonic hedgehog permits pancreatic development. Genes Dev 1998;12:1705–1713. doi:10.1101/gad.12.11.1705.
Furukawa M, Eto Y, Kojima I. Expression of immunoreactive activin A in fetal rat pancreas. Endocrinol Jpn 1995;42:63–68.
Sanvito F, Herrera PL, Huarte J, Nichols A, Montesano R, Orci L, Vassalli JD. TGF-b1 influences the relative development of the exocrine and endocrine pancreas in vitro. Development 1994;120:3451–3462.
Miralles F, Czernichow P, Scharfmann R. Follistatin regulates the relative proportions of endocrine versus exocrine tissue during pancreatic development. Development 1998;125:1017–1024.
Mashima H, Ohnishi H, Wakabayashi K, Mine T, Miyagawa J, Hanafusa T, Seno M, Yamada H, Kojima I. Betacellulin and activin A coordinately convert amylase-secreting pancreatic AR42J cells into insulin-secreting cells. J Clin Invest 1996;97:1647–1654. doi:10.1172/JCI118591.
Zhang YQ, Zhang H, Maeshima A, Kurihara H, Miyagawa J, Takeuchi T, Kojima I. Up-regulation of the expression of activins in the pancreatic duct by reduction of the β-cell mass. Endocrinology 2002;143:3540–3547. doi:10.1210/en.2002-220089.
Shing Y, Christofori G, Hanahan D, Ono Y, Sasada R, Igarashi K, Folkman J. Betacellulin: a mitogen from pancreatic β-cell tumors. Science 1993;259:1604–1607. doi:10.1126/science.8456283.
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999;402:656–660. doi:10.1038/45230.
Wierup N, Svensson H, Mulder H, Sundler F. The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regul Pept 2002;107:63–69. doi:10.1016/S0167-0115(02)00067-8.
Wierup N, Yang S, McEvilly RJ, Mulder H, Sundler F. Ghrelin is expressed in a novel endocrine cell type in devoloping rat islets and inhibits insulin secretion from INS-1 (832/13). J Histochem Cytochem 2004;52:301–310.
Wierup N, Sundller F. Cirulating levels of ghrelin in human fetus. Eur J Endocrinol 2004;150:405. doi:10.1530/eje.0.1500405.
Dezaki K, Hosoda H. Endogenous ghrelin in pancreatic istets resricts insulin release by attenuating Ca 2+ signaling in β-cells. Diabetes 2004;53:3142–3151. doi:10.2337/diabetes.53.12.3142.
Prado CL, Pugh-Bernard AE, Elghazi L, Sosa-Pineda B, Sussel L. Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. Proc Natl Acad Sci U S A 2004;101:2924–2929. doi:10.1073/pnas.0308604100.
Bonnes-Weir S, Trent DF, Weir GC. Partial pancreatectomy in the rat and subsequent defect in glucose-induced insulin release. J Clin Invest 1983;71:1544–1553. doi:10.1172/JCI110910.
Li L, Seno M, Yamada H, Kojima I. Betacellulin improves glucose metabolism by promoting conversion of intraislet precursor cells to β cells in streptozotocin-treated mice. Am J Physiol Endocrinol Metab 2003;285:E577–E583.
Rovertson RP. Islet transplantation as a treatment for diabetes—a work in progress. N Engl J Med 2000;350:694–705. doi:10.1056/NEJMra032425.
Shapiro AMJ, Lakey JRT, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000;27:230–238. doi:10.1056/NEJM200007273430401.
Ryan EA, Lakay JRT, Paty BW, Imes S, Korbutt GS, Kneteman NM, Bigam D, Rajotte RV, Shapiro AMJ. Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes 2002;51:2148–2157. doi:10.2337/diabetes.51.7.2148.
Brockenbrough JS, Weir GC, Bonner-Weir S. Discordance of exocrine and endocrine growth after 90% pancreatectomy in rats. Diabetes 1988;37:232–236. doi:10.2337/diabetes.37.2.232.
Wierup N, Sundler F. Ultrastructure of islet cells in human fetus. Cell Tissue Res 2005;319:423–428. doi:10.1007/s00441-004-1044-x.
Dezaki K, Sone H, Koizumi M, Nakata M, Kakei M, Nagai H, Hosoda H, Kangawa K, Yada T. Blockade of pancreatic islet-derived ghrelin enhances insulin secretion to prevent high-fat diet-induced glucose intolerance. Diabetes 2006;55:3486–3493. doi:10.2337/db06-0878.
Granata R, Settanni F, Trovato L, Destefanis S, Gallo D, Martinetti M, Ghigo E, Muccioli G. Unacylated as well as acylated ghrelin promotes cell survival and inhibit apoptosis in HIT-T15 pancreatic beta-cells. J Endocrinol Invest 2006;29:RC19–RC22.
Granata R, Settanni F, Biancone L, Trovato L, Nano R, Bertuzzi F, Destefanis S, Annunziata M, Martinetti M, Catapano F, Ghè C, Isgaard J, Papotti M, Ghigo E, Muccioli G. Acylated and unacylated ghrelin promote proliferation and inhibit apoptosis of pancreatic beta-cells and human islets: involvement of 3′,5′-cyclic adenosine monophosphate/protein kinase A, extracellular signal-regulated kinase 1/2, and phosphatidyl inositol 3-Kinase/Akt signaling. Endocrinology 2007;148:512–529. doi:10.1210/en.2006-0266.
Irako T, Akamizu T, Hosoda H, Iwakura H, Ariyasu H, Tojo K, Tajima N, Kangawa K. Ghrelin prevents development of diabetes at adult age in streptozotocin-treated newborn rats. Diabetologia 2006;49:1264–1273. doi:10.1007/s00125-006-0226-3.
Doi A, Shono T, Nishi M, Furuta H, Sasaki H, Nanjo K. IA-2beta, but not IA-2, is induced by ghrelin and inhibits glucose-stimulated insulin secretion. Proc Natl Acad Sci U S A 2006;103:885–890. doi:10.1073/pnas.0502470102.
Asakawa A, Inui A, Kaga T. Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut 2003;52:547–552. doi:10.1136/gut.52.7.947.
Acknowledgments
This study was supported by a grant from The Scientific and Technological Research Council of Turkey Ankara, Turkey. Project No: SBAG-HD-154 (106S231). This study was presented at the Turkish National Surgical Congress, May 2008, Antalya, Turkey
Author information
Authors and Affiliations
Corresponding author
Additional information
Kerem M and Salman B contributed equally to this work; Kerem M, Salman B, and Bedirli A designed experiments; Kerem M, Salman B, Pasaoglu H, Ozsoy S, Haziroglu R, and Yilmaz Tu performed experiments; Kerem M, Salman B, and Bedirli A analyzed data; Kerem M, Salman B, and Bedirli A wrote the paper.
Rights and permissions
About this article
Cite this article
Kerem, M., Salman, B., Ozsoy, S. et al. Exogenous Ghrelin Enhances Endocrine and Exocrine Regeneration in Pancreatectomized Rats. J Gastrointest Surg 13, 775–783 (2009). https://doi.org/10.1007/s11605-008-0778-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-008-0778-2