Abstract
Background
Enoxaparin is an important molecule which had been using in prophylaxis and treatment of deep venous thrombosis. Also, it is showed that it prevents postsurgical peritoneal adhesions in rats. It is aimed to evaluate its effects on gastrointestinal wound healing.
Methods
Thirty Wistar albino rats were divided into three groups as control, subcutan, and intraperitoneal enoxaparin groups. Left colon anastomoses were performed. On postoperative seventh day, anastomotic healing was evaluated by measuring anastomotic bursting pressure, tissue hydroxyproline levels, and histopathological examination.
Results
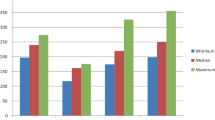
The anastomotic bursting pressure was highest in subcutan enoxaparin group (p < 0.001), intraperitoneal enoxaparin group (p < 0.01) came the second, and the control group has the worst value. The hydroxyproline results were found nearly similar to the bursting pressure values (subcutan (p < 0.001) > intraperitoneal (p < 0.05) > control). Neovascularization in subcutan group (p < 0.001) has a statistically significant difference to other groups.
Conclusion
Enoxaparin did not interfere with colonic anastomotic resistance but improved the intestinal wound healing.


Similar content being viewed by others
References
Ergul E, Korukluoglu B. Peritoneal adhesions: facing the enemy. Int J Surg. 2008;6(3):253–260. doi:10.1016/j.ijsu.2007.05.010.
Celik A, Ucar AE, Ergul E, Bekar ME, Kusdemir A. Which is most effective in prevention of postoperative intraperitoneal adhesions—Methylene blue, low molecular weight heparin or vitamin E: an experimental study in rats. Internet J Surg 2008;15(1).
Christensen H, Langfelt S, Laurberg S. Bursting strength of experimental colonic anastomoses. A methodological study. Eur Surg Res 1993;25:38–45. doi:10.1159/000129255.
Uzunkoy A, Akinci OF, Coskun A, Aslan O, Kocyigit A. Effects of antiadhesive agents on the healing of intestinal anastomosis. Dis Colon Rectum 2000;43:370–375. doi:10.1007/BF02258304.
Mansson P, Zhang XW, Jeppsson B, Thorlacius H. Anastomotic healing in the rat colon: comparison between a radiological method, breaking strength and bursting pressure. Int J Colorectal Dis 2002;17:420–425. doi:10.1007/s00384-002-0392-9.
Hendriks T, Mastboom WJ. Healing of experimental intestinal anastomoses. Parameters for repair. Dis Colon Rectum 1990;33:891–901. doi:10.1007/BF02051930.
Jonsson K, Jiborn H, Zederfeldt B. Mechanical and biochemical alterations in the intestinal wall adjacent to an anastomosis. Am J Surg 1986;151:387–90. doi:10.1016/0002-9610(86)90474-5.
Bergman I, Loxley R. Two improved and simplified methods for the spectrophotometric determination of hydroxyproline. Anal Chem 1963;35:1961–1965. doi:10.1021/ac60205a053.
Hunt TK, Mueller RV. Wound healing. In Way LW, ed. Current surgical diagnosis and treatment, 10th ed. New Jersey: Appleton and Lange, 1994, pp 80–93.
Ehrlich HP, Tarver H, Hunt TK. Effects of vitamin A and glucocorticoids upon inflammation and collagen synthesis. Ann Surg 1973;177:222–227. doi:10.1097/00000658-197302000-00017.
Martens MF, Hendriks T, de Koning HJ. Postoperative changes in collagen synthesis in intestinal anastomoses of the rat: differences between small and large bowel. Gut 1991;32:1482–1487. doi:10.1136/gut.32.12.1482.
Ferrao AV, Mason RM. The effect of heparin on cell proliferation and type-I collagen synthesis by adult human dermal fibroblasts. Biochim Biophys Acta 1993;1180:225–230.
Andriuoli G, Mastacchi R, Barbanti M, et al. Comparison of the antithrombotic and hemorrhagic effects of heparin and a new low molecular weigh heparin in the rat. Haemostasis 1985;15:324.
Okutan H, Eroglu E, Karahan N, Aydin A, Tunc B, Candir O, Kutsal A. Comparison of different low-molecular-weight heparins (dalteparin, enoxaparin, nadroparine) and standard heparin in rat venous thrombosis model. Turk J Vasc Surg 2004;13:17–22.
Lynd LD, Goeree R, Crowther MA, O’Brien BJ. A probabilistic cost-effectiveness analysis of enoxaparin versus unfractionated heparin for the prophylaxis of deep-vein thrombosis following major trauma. Can J Clin Pharmacol 2007;14:215–226.
Sahin Y, Saglam A. Synergistic effects of carboxymethylcellulose a low molecular weight heparin in reducing adhesion formation in the rat uterine horn model. Acta Obstet Gynecol Scand 1994;73:70–73. doi:10.3109/00016349409013399.
Arikan S, Adas G, Barut G, Toklu AS, Kocakusak A, Uzun H, Kemik O, Daduk Y, Aydin S, Purisa S. An evaluation of low molecular weight heparin and hyperbaric oxygen treatment in the prevention of intra-abdominal adhesions and wound healing. Am J Surg 2005;189:155–160. doi:10.1016/j.amjsurg.2004.11.002.
Jorneskog G, Brismar K, Fagrell B. Low molecular weight heparin seems to improve local capillary circulation and healing of chronic foot ulcers in diabetic patients. Vasa 1993;22:137–142.
Sifil A, Mermut C, Yenicerioglu Y, Cavdar C, Gumustekin M, Celik A, Yuksel F, Camsari T. Intraperitoneal and subcutaneous pharmacokinetics of low molecular weight heparin in continuous ambulatory peritoneal dialysis patients. Adv Perit Dial 2003;19:28–30.
Basson MD. Invited research review: cell–matrix interactions in the gut epithelium. Surgery 2003;133:63–67. doi:10.1067/msy.2003.24.
Levine A, Kenet G, Bruck R, Avni Y, Avinoach I, Aeed H, Matas Z, David M, Yayon A. Effect of heparin on tissue binding activity of fibroblast growth factor and heparin binding epidermal growth factor in experimental colitis in rats. Pediatr Res 2002;5:635–640. doi:10.1203/00006450-200205000-00015.
Street JT, McGrath M, O’Regan K, Wakai A, Mc Ginness A, Redmond HPR. Thromboprophylaxis using a low molecular weight heparin delays fracture repair. Clin Orthop Relat Res 2000;381:278–289. doi:10.1097/00003086-200012000-00032.
Michell NP, Lalor P, Langman MJ. Heparin therapy for ulcerative colitis? Effects and mechanisms. Eur J Gastroenterol Hepatol 2001;13:449–456. doi:10.1097/00042737-200104000-00026.
Galvan L. Effects of heparin on wound healing. J Wound Ostomy Continence Nurs 1996;23:224–226. doi:10.1016/S1071-5754(96)90095-9.
Folkman J. Regulation of angiogenesis: a new function of heparin. Biochem Pharmacol 1985;34:905–909. doi:10.1016/0006-2952(85)90588-X.
Terranova VP, Diflorio R, Lyall RM, Hic S, Friesel R, Maciag T. Human endothelial cells are chemotactic to endothelial cell growth factor and heparin. J Cell Biol 1985;101:2330–2334. doi:10.1083/jcb.101.6.2330.
Piazuelo E, Jiemenez P, Lanas A, Garcia A, Esteva F, Sainz R. Platelet-derived growth factor and epidermal growth factor play a role in human colonic fibroblastic repair. Eur Surg Res 2000;32:191–196. doi:10.1159/000008762.
Jorneskog G, Brismar K, Fagrell B. Low molecular weight heparin seems to improve local capillary circulation and healing of chronic foot ulcers in diabetic patients. Vasa 1993;22:137–142.
Saliba MJ. The effects and uses of heparin in the care of burns that improves treatment and enhances the quality of life. Acta Chir Plast 1997;39:13–16.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ergul, E., Ozgun, Y.M., Kiyak, G. et al. Does Low Molecular Weight Heparin Impair Anastomotic Wound Healing?. J Gastrointest Surg 13, 798–803 (2009). https://doi.org/10.1007/s11605-008-0771-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-008-0771-9