Abstract
Purpose
This study aimed to investigate the uptake characteristics of 18F-fluoromisonidazole (FMISO), in mutant-type isocitrate dehydrogenase (IDH-mutant, grade 3 and 4) and wild-type IDH (IDH-wildtype, grade 4) 2021 WHO classification adult-type diffuse gliomas.
Materials and methods
Patients with grade 3 and 4 adult-type diffuse gliomas (n = 35) were included in this prospective study. After registering 18F-FMISO PET and MR images, standardized uptake value (SUV) and apparent diffusion coefficient (ADC) were evaluated in hyperintense areas on fluid-attenuated inversion recovery (FLAIR) imaging (HIA), and in contrast-enhanced tumors (CET) by manually placing 3D volumes of interest. Relative SUVmax (rSUVmax) and SUVmean (rSUVmean), 10th percentile of ADC (ADC10pct), mean ADC (ADCmean) were measured in HIA and CET, respectively.
Results
rSUVmean in HIA and rSUVmean in CET were significantly higher in IDH-wildtype than in IDH-mutant (P = 0.0496 and 0.03, respectively). The combination of FMISO rSUVmean in HIA and ADC10pct in CET, that of rSUVmax and ADC10pct in CET, that of rSUVmean in HIA and ADCmean in CET, were able to differentiate IDH-mutant from IDH-wildtype (AUC 0.80). When confined to astrocytic tumors except for oligodendroglioma, rSUVmax, rSUVmean in HIA and rSUVmean in CET were higher for IDH-wildtype than for IDH-mutant, but not significantly (P = 0.23, 0.13 and 0.14, respectively). The combination of FMISO rSUVmean in HIA and ADC10pct in CET was able to differentiate IDH-mutant (AUC 0.81).
Conclusion
PET using 18F-FMISO and ADC might provide a valuable tool for differentiating between IDH mutation status of 2021 WHO classification grade 3 and 4 adult-type diffuse gliomas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The radiotracer 18F-fluoromisonidazole (FMISO) accumulates in hypoxic viable cells after reduction reactions in the absence of oxygen. PET using 18F-FMISO allows the detection of hypoxia associated with the rapid depletion of nutrients that occurs with the abnormal proliferation of tumor cells seen in glioma [1, 2]. Hypoxia is associated with resistance to radiotherapy and chemotherapy in gliomas, and is related to the outcomes of glioma therapies [3]. Despite the importance of clarifying the extent of hypoxia in gliomas, common imaging modalities cannot clearly identify hypoxia in gliomas.
Isocitrate dehydrogenase (IDH) mutation is known to affect the prognosis of patients with glioma [4,5,6], and the knowledge of IDH mutation has been incorporated into 2021 WHO classification of brain tumors [7]. Prediction of IDH mutation by imaging would facilitate the optimization of therapeutic strategies for gliomas. Previous reports have demonstrated that 2-hydroxyglutarate (2-HG), which accumulates in IDH-mutated gliomas, can be detected by magnetic resonance spectroscopy [8]. On the other hand, PET probes have been reported to potentially allow prediction of IDH mutation status. Recent studies have found significant associations between 18F-fluoro-ethyl-tyrosine (18F-FET) PET results and IDH mutation status [9,10,11]. Another recent paper investigated the association of 3'-deoxy-3'-18F-fluorothymidine (18F-FLT) PET and 18F-FMISO PET, as well as relative cerebral blood volume in 31 patients with glioblastoma [12].
As 18F-FMISO PET is known to be useful in differentiating glioma grades [13], we hypothesized that there might be some association between IDH mutation status and 18F-FMISO uptake in glioma. The present study aimed to investigate the characteristics of 18F-FMISO uptake by 2021 WHO classification grade 3 and 4 glioma in terms of IDH mutation status.
Materials and methods
Patients
The institutional ethics committee approved this prospective study. Patients who were suspected intracranial brain lesions were enrolled in this study between September 2015 and March 2018, and written informed consent was obtained from each patient. In cases where the patient could not provide a signature, another family member provided informed consent instead. Tumors were included or excluded according to 2021 WHO classifications [7]. Figure 1 shows the inclusion and exclusion criteria. First, we included patients with histopathological diagnoses of 2021 WHO classifications grade 3 and 4 glioma (n = 35), which includes: (a) Glioblastoma, IDH-wildtype, grade 4 (n = 22); (b) Astrocytoma, IDH-mutant, grade 3, 4 (n = 9); (c) Oligodendroglioma, IDH-mutant and 1p/19q-codeleted, grade 3 (n = 4). Second, patients who were histopathologically diagnosed with other brain tumors were excluded from our study (n = 6). Third, glioma, not otherwise specified (NOS) were excluded from our study (n = 3). Fourth, grade 2 gliomas were excluded from our study (Astrocytoma, IDH-mutant, n = 4; Oligodendroglioma, IDH-mutant and 1p/19q-codeleted, n = 2). Fifth, IDH testing was not performed on four patients because histopathology was performed between 2011 and 2014 or because the specimens were not in suitably good condition, so those four patients were excluded from the study (n = 4). IDH testing was performed with an immunohistochemistry assay.
A flowchart of inclusion and exclusion criteria for this study. A total of 35 patients were included in this study (Glioblastoma, IDH-wildtype, n = 22; Astrocytoma, IDH-mutant, grade 3, 4, n = 9; Oligodendroglioma, IDH-mutant, and 1p/19q-codeleted, grade 3, n = 4). Note that patients who were histopathologically diagnosed with other brain tumors were excluded from our study (n = 6) (*). Five of them were histopathologically diagnosed as schwannoma 2, metastatic tumor 1, central neurocytoma 1, ependymoma 1. One patient was clinically diagnosed as pilocytic astrocytoma without surgery at the time of PET scan, but was recently operated due to tumor volume increase, and histopathologically diagnosed as pilocytic astrocytoma
PET protocol
Static PET images of the brain were acquired 4 h after intravenous injection of 350–550 MBq of 18F-FMISO. All images were acquired using a PET/CT scanner (Discovery IQ; GE Healthcare, Milwaukee, WI) with 5 circular BGO (Bi4Ge3O12) detectors. Low-dose CT was obtained as follows: 120 kVp; transaxial field of view (FOV), 500 × 500 mm; matrix size, 512 × 512; slice thickness, 3.75 mm. Next, emission scan was performed for 15 min and images were reconstructed with attenuation correction using CT data: transaxial FOV, 250 × 250 mm; matrix size, 192 × 192; axial FOV, 250 mm; voxel size, 1.3 × 1.3 × 3.3 mm; post-filtering at 2 mm full-width at half-maximum, VUE Point HD (3D ordered subsets expectation–maximization, OSEM), point spread function (subsets, 12; iterations, 4; filter cutoff, 2.0 mm).
MR protocol
Brain MRI was conducted using a 3-T MRI unit (MAGNETOM Prisma or Skyra; Siemens Healthineers, Erlangen, Germany) with a 64-channel head/neck coil or a 32-channel head coil, including the following image sequences: non-enhanced (NE) and contrast-enhanced (CE) 3-dimensional (3D) T1-weighted imaging (T1WI): TR/TE, 6.0/2.3 ms; flip angle (FA), 15º; FOV, 230 × 230 mm2; resolution 0.9 × 0.9 mm2; slice thickness, 0.9 mm. Fluid-attenuated inversion recovery (FLAIR): TR/TE/TI, 12,000/100/2760 ms; FA, 120º; FOV, 220 × 192 mm2; resolution 0.69 × 0.69 mm2; slice thickness, 4 mm. Apparent diffusion coefficient (ADC) map was created from diffusion-weighted imaging (b = 0, 1000 s/mm2): TR/TE, 5000/77 ms; FA, 90º; FOV 220 × 220 mm2; resolution, 0.69 × 0.69 mm2; slice thickness, 4 mm. Gadolinium-based contrast agent (GBCA, 0.1 mmol/kg) was administered intravenously for CE-T1WI.
Image processing and parameter calculation
Registration
Images from 18F-FMISO PET, FLAIR, ADC map, and CE 3D T1WI were registered to NE 3D T1WI images using SPM12 (https://www.fil.ion.ucl.ac.uk/spm). Images from NE 3D T1WI were segmented into gray matter, white matter, and other parts using SPM12.
Definition of volume of interest (VOI)
Two VOIs were manually placed by a board-certified radiologist with 8 years of experience in neuroradiology, using ITK-SNAP software (https://www.itksnap.org) [14] and approved by another board-certified radiologist with 22 years of experience in neuroradiology. (a) Hyperintense areas on FLAIR imaging (HIA) were defined as areas of hyperintensity around and inside the tumor on FLAIR imaging. (b) Contrast-enhanced tumors (CET) were defined as areas of tumor enhancement on CE T1WI. Areas of central hypointensity on CE T1WI were considered to represent regions of central necrosis and were excluded from among VOIs of CET. Hyperintense areas on NE T1WI were considered to represent hemorrhagic lesions and were removed from among the VOIs of CET. Representative VOIs are shown in Fig. 2. Cerebellar cortical VOIs were created for reference using segmented cerebellar cortices.
Representative VOIs for the case with Glioblastoma, IDH-wildtype are shown. FMISO (top), CE T1WI (middle row), FLAIR (bottom). Hyperintense areas on FLAIR imaging (HIA), representing areas of hyperintensity around and inside the tumor on FLAIR imaging (green), contrast-enhancing tumors (CET), representing tumor areas limited by the area of hyperintensity on CE T1WI (red)
Data analysis
Relative standardized uptake value (SUV) of 18F-FMISO PET images was calculated as follows: SUVmax, SUVmean in HIA, and SUVmean in CET were divided by the SUVmean of cerebellar cortical VOIs, respectively. This resulted in rSUVmax and rSUVmean in HIA, and rSUVmean in CET.
We measured ADC10pct in HIA, ADCmean in HIA, ADC10pct in CET, and ADCmean in CET using the ADC map of MRI images [15, 16]. MANGO software (Research Imaging Institute, UTHSCSA, http://ric.uthscsa.edu/mango/) was used for these measurements.
We performed the following analyses to determine IDH mutation status of glioma.
IDH-mutant vs. IDH-wildtype
We compared rSUVmax and rSUVmean in HIA, rSUVmean in CET, ADC10pct and ADCmean in HIA, and ADC10pct and ADCmean in CET between IDH-mutant and IDH-wildtype in terms of following: A) comparisons between IDH-mutant and IDH-wildtype in all patients (n = 35); B) in patients in astrocytic tumors (n = 31).
Statistical analysis
We applied the Mann–Whitney U test for measured values that did not follow a normal distribution.
We also performed logistic regression analysis with the above-mentioned rSUV and ADC, followed by receiver operating characteristic (ROC) curve analysis. Areas under the curve (AUCs) were calculated with optimal cutoff, sensitivity, and specificity in terms of following: (A) determination of IDH mutation status in all patients; (B) determination of IDH mutation status in patients in astrocytic gliomas; AUCs were compared with DeLong test.
All statistical analyses were performed using JMP version 15 software (SAS Institute, Cary, NC, United States). Values of P < 0.05 were considered significant.
Results
Patients
A total of 35 patients were included in this study. The demographic characteristics of patients are shown in Table 1 and Fig. 1. rSUV and ADC in IDH-mutant and IDH-wildtype are shown in Table 2. No registration error was observed. Representative images are shown in Fig. 3.
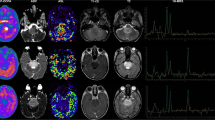
Three representative cases are shown. A 60-year-old man with Glioblastoma, IDH-wildtype, grade 4 (top); A 67-year-old woman with Astrocytoma, IDH-mutant, grade 4 (second row); A 24-year-old man with Oligodendroglioma, IDH-mutant, and 1p/19q-codeleted, grade 3 (bottom). FMISO-PET shows prominent uptake in glioblastoma, IDH-wildtype, and moderate uptake in Astrocytoma, IDH-mutant, grade 4. Meanwhile, FMISO-PET shows low uptake in Oligodendroglioma, IDH-mutant, and 1p/19q-codeleted
Comparisons between IDH-mutant and IDH-wildtype in all patients (n = 35)
rSUVmean in HIA and rSUVmean in CET were significantly higher for IDH-wildtype than for IDH-mutant (P = 0.0496 and 0.03, respectively) (Fig. 4). rSUVmax were higher for IDH-wildtype than for IDH-mutant but not significantly (P = 0.06).
Comparison between IDH-mutant (n = 13) and IDH-wildtype (n = 22) in all patients. The rSUVmean in HIA (b), and rSUVmean in CET (c) are significantly higher for IDH-wildtype than for IDH-mutant. The rSUVmax (a) tend to be higher for IDH-wildtype than for IDH-mutant, but not significantly. The ADC10pct in HIA (d) and ADCmean in HIA (f) tend to be lower for IDH-wildtype than for IDH-mutant, but not significantly. No significant differences were found in ADC10pct in CET (e) and ADCmean in CET (g). Asterisks (*) represent statistically significant differences
ADC10pct in HIA and ADCmean in HIA were lower for IDH-wildtype than for IDH-mutant but not significantly (P = 0.24 and 0.16, respectively). No significant differences were found in ADC10pct in CET or ADCmean in CET (P = 0.98 and 0.54, respectively).
Comparisons between IDH-mutant and IDH-wildtype in patients in astrocytic tumors (n = 31)
rSUVmax, rSUVmean in HIA and rSUVmean in CET were higher for IDH-wildtype than for IDH-mutant, but not significantly (P = 0.23, 0.13 and 0.14, respectively) (Fig. 5).
Comparison between IDH-mutant (n = 9) and IDH-wildtype (n = 22) in astrocytic tumors. The rSUVmax (a), rSUVmean in HIA (b) and rSUVmean in CET (c) were higher for IDH-wildtype than for IDH-mutant, but not significantly. The ADC10pct in HIA (d) and ADCmean in HIA (f) were lower for IDH-wildtype than for IDH-mutant but not significantly. No significant differences were found in ADC10pct in CET (e) or ADCmean in CET (g)
ADC10pct in HIA and ADCmean in HIA were lower for IDH-wildtype than for IDH-mutant but not significantly (P = 0.45 and 0.23, respectively). No significant differences were found in ADC10pct in CET or ADCmean in CET (P = 0.82 and 0.63, respectively).
ROC curve analysis
ROC curve analysis was performed using logistic regression analysis with rSUV and ADC to determine glioma IDH mutations status in all patients (n = 35) (Fig. 6a) and in astrocytic tumors (n = 31) (Fig. 6b). AUCs of all parameters are shown in Supplemental Tables 1 and 2.
ROC analysis was performed for 2 groups. The highest three AUCs were shown for each group. a Prediction of IDH mutation status in all patients (n = 35): Model 1A, rSUVmean in HIA and ADC10pct in CET (AUC, 0.80); Model 1B, rSUVmax and ADC10pct in CET (AUC, 0.80); Model 1C, rSUVmean in HIA and ADCmean in CET (AUC, 0.80). b Prediction of IDH mutation status in the patients of astrocytic tumors (n = 31): Model 2A, rSUVmean in HIA and ADC10pct in CET (AUC, 0.81); Model 2B, rSUVmax and ADC10pct in CET (AUC, 0.79); Model 2C, rSUVmean in HIA and ADCmean in CET (AUC, 0.76)
The best three models for IDH mutation status in all patients (n = 35) were Model 1A, 1B and 1C. Model 1A, rSUVmean in HIA and ADC10pct in CET (AUC, 0.80). The optimal cutoffs (rSUVmean in HIA 1.02; ADC10pct in CET 1.07) offered 75.0% sensitivity and 77.8% specificity; Model 1B using rSUVmax and ADC10pct in CET (AUC, 0.80). The optimal cutoffs (rSUVmax 2.19; ADC10pct in CET 1.07) offered 83.3% sensitivity and 61.1% specificity; Model 1C, rSUVmean HIA and ADCmean in CET (AUC, 0.80). The optimal cutoffs rSUVmean HIA 1.02; ADCmean in CET 1.55) offered 75.0% sensitivity and 77.8% specificity (Fig. 6a). DeLong test showed no statistical significance in AUC was observed between each model (Supplemental Table 3).
The best model for IDH mutation status in astrocytic tumor patients (n = 31) was Model 2A, using rSUVmean in HIA and ADC10pct in CET (AUC, 0.81). The optimal cutoffs (rSUVmean in HIA 1.05; ADC10pct in CET 0.82) offered 66.7% sensitivity and 88.9% specificity; Model 2B using rSUVmax and ADC10pct in CET (AUC, 0.79). The optimal cutoffs (rSUVmax 2.81; ADC10pct in CET 0.62) offered 55.6% sensitivity and 94.4% specificity; Model 2C, rSUVmean HIA and ADCmean in CET (AUC, 0.76). The optimal cutoffs rSUVmean HIA 1.05; ADCmean in CET 1.28) offered 55.6% sensitivity and 83.3% specificity (Fig. 6b). DeLong test showed no statistical significance in AUC was observed between each model (Supplemental Table 3).
Discussion
We were able to differentiate IDH mutation status using rSUVmean in HIA and ADC10pct in CET, with AUC of 0.80 in all patients, and AUC of 0.81 in the patients with astrocytic tumors, according to 2021 WHO classification. Obviously, FMISO does not directly reflect IDH mutation status. A recent study revealed that multidrug-resistant protein 1 (MRP1) inhibitors increase 18F-FMISO accumulation in hypoxic cells. This suggests that 18F-FMISO PET imaging is affected by MRP1 inhibitors independent of the state of hypoxia [17]. FMISO uptake is known to reflect hypoxic circumstances in tissues, and increased tumor aggressiveness induces greater hypoxia inside the tumor [18,19,20,21]. IDH mutation is considered to offer a strong predictor of less-aggressive glioma [22,23,24], and FMISO would thus indirectly reflect IDH mutation status.
In the present study, no significant difference in ADC was apparent between IDH-wildtype and IDH-mutant. However, diagnostic performance increased after combining ADC with SUV. Our study demonstrated that rSUVmean in HIA and ADC10pct in CET can differentiate IDH mutation status with high diagnostic ability in grade 3, 4 gliomas by 2021 WHO classification (AUC = 0.80, in all patients; AUC = 0.81, in astrocytic tumors, respectively). In the literature, detection of IDH mutation status was possible in grade 2 and 3 glioma of 2016 WHO classification, using the ratio of ADCmean to ADC of normal-appearing white matter (AUC 0.83) [25], ADC ratio (AUC 0.95) [26], minimum ADC (ADCmin) (AUC 0.87), and relative ADCmin (AUC 0.84) [27]. Meanwhile, in grade 3, 4 gliomas, IDH mutation status was able to be differentiated by ADCmean (AUC 0.71), ADC10pct (AUC 0.71) from histogram study [28], and ratio of ADCmin to normal white matter (AUC = 0.70) in grade 4 glioma [29]. Previous study also showed that, FMISO tumor–blood SUV ratio (TBR) could differentiate IDH-mutant type from IDH- wildtype in grade 3, 4 gliomas (AUC = 0.78 in all patients, AUC = 0.76 in astrocytic tumors) [30]. Our results were comparable to the those of previous studies.
IDH mutation status has some association with other PET tracers. The ratios of the SUVmax of the tumors to the SUVmean of the contralateral cortex (T/N ratios) of FLT-PET/CT can be used to determine the IDH mutation status with an AUC of 0.911; The T/N ratios of 11C-methionine (MET) methionine can be used to determine the IDH mutation status with an AUC of 0.727 [31]. Based on 2016 WHO classification, the differences in mean 18F-FLT tumor-normal tissue ratio (TNR) and 18F-FMISO TBR were significant between GBM and other glioma subtypes (P < 0.001); and regarding the comparison between Gd-T1WI volumes and 18F-FLT MTVs or 18F-FMISO MTVs, previous study identified significant differences between IDH-wildtype and IDH-mutant or 1p19q-codeletion (P < 0.01) [32]. The percentage difference between the standard biological tumor volume (BTV) on standard summation images and BTV on early summation images could differentiate IDH mutation status with an AUC of 0.83 using 18F-FET PET [10]. Time-to-peak value in a dynamic 18F-FET PET study also showed good diagnostic performance for IDH mutation status in gliomas (AUC 0.75) [33]. While 3D-VOIs were manually created for HIA and CET in our study, as in a previous study [34], 2-dimensional regions of interest (2D-ROI) were used in most studies examining 18F-FMISO PET. Our study registered images from PET and MRI, and 3D-segmented cerebellar cortices were also used as references to calculate relative SUV, although 2D-ROIs were used as reference for 18F-FMISO uptakes in previous articles [18, 34, 35]. 3D-VOIs for HIA may underestimate rSUVmean of 18F-FMISO uptake because 3D-VOIs for HIA are larger than those for CET. However, rSUVmax can compensate such underestimation in HIA, since physiological 18F-FMISO uptake is low and homogeneous in the brain parenchyma compared with other amino acid PET tracers.
Some limitations need to be acknowledged in this study. First, the number of patients enrolled was small (n = 35). More patients need to be enrolled to confirm the present results. Second, biodistribution of 18F-FMISO has not been evaluated in this study. 18F-FMISO is relative lipophilic and diffuses through cell membranes, and mild uptake of 18F-FMISO is seen in normal tissue [36]. In addition, the previous study had performed dynamic 18F-FMISO and dynamic 15O-H2O PET in brain tumors to measure tumor hypoxia and perfusion, and increased 18F-FMISO tumor retention at late scan time was found predominantly in glioblastoma, but not found in meningiomas, which lacks the blood brain barrier (BBB) [35]. Their data suggested that late 18F-FMISO PET images obtained 4 h after the injection provide a spatial description of hypoxia in brain tumors that is independent of BBB disruption and tumor perfusion. Third, dynamic susceptibility contrast perfusion weighted imaging (DSC-PWI), which is beneficial in differentiation IDH-wildtype glioma and IDH-mutant glioma, was not used in this study. IDH mutation status of grade 2 and 3 gliomas of 2016 WHO classification can be differentiated by relative CBVmax with an AUC of 0.82 [27]. IDH mutation status in glioblastoma could be differentiated by rCBVmean with AUC of 0.886 [37]. IDH mutation status could be differentiated using Visually AcceSAble Rembrandt Images (VASARI) MRI feature set in grade 2 and 3 gliomas, grade 2 glioma only, and grade 3 glioma only with AUCs of 0.78, 0.83 and 0.87, respectively [38].
Conclusions
In conclusion, PET using 18F-FMISO and ADC might provide a valuable tool for differentiating IDH mutation status of 2021 WHO classification grade 3 and 4 adult-type diffuse glioma.
References
Valable S, Petit E, Roussel S, Marteau L, Toutain J, Divoux D, et al. Complementary information from magnetic resonance imaging and (18)F-fluoromisonidazole positron emission tomography in the assessment of the response to an antiangiogenic treatment in a rat brain tumor model. Nucl Med Biol. 2011;38:781–93.
Swanson KR, Chakraborty G, Wang CH, Rockne R, Harpold HL, Muzi M, et al. Complementary but distinct roles for MRI and 18F-fluoromisonidazole PET in the assessment of human glioblastomas. J Nucl Med. 2009;50:36–44.
Bekaert L, Valable S, Lechapt-Zalcman E, Ponte K, Collet S, Constans JM, et al. [18F]-FMISO PET study of hypoxia in gliomas before surgery: correlation with molecular markers of hypoxia and angiogenesis. Eur J Nucl Med Mol Imaging. 2017;44:1383–92.
Han S, Liu Y, Cai SJ, Qian M, Ding J, Larion M, et al. IDH mutation in glioma: molecular mechanisms and potential therapeutic targets. Br J Cancer. 2020;122:1580–9.
Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013;19:764–72.
Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–12.
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23:1231–51.
Choi C, Ganji SK, DeBerardinis RJ, Hatanpaa KJ, Rakheja D, Kovacs Z, et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med. 2012;18:624–9.
Blanc-Durand P, Van Der Gucht A, Verger A, Langen KJ, Dunet V, Bloch J, et al. Voxel-based 18F-FET PET segmentation and automatic clustering of tumor voxels: a significant association with IDH1 mutation status and survival in patients with gliomas. PLoS ONE. 2018;13:e0199379.
Unterrainer M, Winkelmann I, Suchorska B, Giese A, Wenter V, Kreth FW, et al. Biological tumour volumes of gliomas in early and standard 20–40 min 18F-FET PET images differ according to IDH mutation status. Eur J Nucl Med Mol Imaging. 2018;45:1242–9.
Verger A, Stoffels G, Bauer EK, Lohmann P, Blau T, Fink GR, et al. Static and dynamic 18 F-FET PET for the characterization of gliomas defined by IDH and 1p/19q status. Eur J Nucl Med Mol Imaging. 2018;45:443–51.
Collet S, Guillamo JS, Berro DH, Chakhoyan A, Constans JM, Lechapt-Zalcman E, et al. Simultaneous mapping of vasculature, hypoxia, and proliferation using dynamic susceptibility contrast MRI, (18)F-FMISO PET, and (18)F-FLT PET in relation to contrast enhancement in newly diagnosed glioblastoma. J Nucl Med. 2021;62:1349–56.
Kanoto M, Kirii K, Hiraka T, Toyoguchi Y, Sugai Y, Matsuda K, et al. Correlation between hypoxic area in primary brain tumors and WHO grade: differentiation from malignancy using 18F-fluoromisonidazole positron emission tomography. Acta Radiol. 2018;59:229–35.
Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–28.
Burth S, Kickingereder P, Eidel O, Tichy D, Bonekamp D, Weberling L, et al. Clinical parameters outweigh diffusion- and perfusion-derived MRI parameters in predicting survival in newly diagnosed glioblastoma. Neuro Oncol. 2016;18:1673–9.
Wen Q, Jalilian L, Lupo JM, Molinaro AM, Chang SM, Clarke J, et al. Comparison of ADC metrics and their association with outcome for patients with newly diagnosed glioblastoma being treated with radiation therapy, temozolomide, erlotinib and bevacizumab. J Neurooncol. 2015;121:331–9.
Shimizu Y, Nakai Y, Watanabe H, Iikuni S, Ono M, Saji H, et al. Increased [(18)F]FMISO accumulation under hypoxia by multidrug-resistant protein 1 inhibitors. EJNMMI Res. 2021;11:9.
Hirata K, Terasaka S, Shiga T, Hattori N, Magota K, Kobayashi H, et al. (1)(8)F-Fluoromisonidazole positron emission tomography may differentiate glioblastoma multiforme from less malignant gliomas. Eur J Nucl Med Mol Imaging. 2012;39:760–70.
Cher LM, Murone C, Lawrentschuk N, Ramdave S, Papenfuss A, Hannah A, et al. Correlation of hypoxic cell fraction and angiogenesis with glucose metabolic rate in gliomas using 18F-fluoromisonidazole, 18F-FDG PET, and immunohistochemical studies. J Nucl Med. 2006;47:410–8.
Szeto MD, Chakraborty G, Hadley J, Rockne R, Muzi M, Alvord EC, et al. Quantitative metrics of net proliferation and invasion link biological aggressiveness assessed by MRI with hypoxia assessed by FMISO-PET in newly diagnosed glioblastomas. Cancer Res. 2009;69:4502–9.
Kawai N, Maeda Y, Kudomi N, Miyake K, Okada M, Yamamoto Y, et al. Correlation of biological aggressiveness assessed by 11C-methionine PET and hypoxic burden assessed by 18F-fluoromisonidazole PET in newly diagnosed glioblastoma. Eur J Nucl Med Mol Imaging. 2011;38:441–50.
Sanson M, Marie Y, Paris S, Idbaih A, Laffaire J, Ducray F, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009;27:4150–4.
Reuss DE, Kratz A, Sahm F, Capper D, Schrimpf D, Koelsche C, et al. Adult IDH wild type astrocytomas biologically and clinically resolve into other tumor entities. Acta Neuropathol. 2015;130:407–17.
Hartmann C, Hentschel B, Tatagiba M, Schramm J, Schnell O, Seidel C, et al. Molecular markers in low-grade gliomas: predictive or prognostic? Clin Cancer Res. 2011;17:4588–99.
Maynard J, Okuchi S, Wastling S, Busaidi AA, Almossawi O, Mbatha W, et al. World health organization grade II/III glioma molecular status: prediction by MRI morphologic features and apparent diffusion coefficient. Radiology. 2020;296:111–21.
Thust SC, Hassanein S, Bisdas S, Rees JH, Hyare H, Maynard JA, et al. Apparent diffusion coefficient for molecular subtyping of non-gadolinium-enhancing WHO grade II/III glioma: volumetric segmentation versus two-dimensional region of interest analysis. Eur Radiol. 2018;28:3779–88.
Xing Z, Yang X, She D, Lin Y, Zhang Y, Cao D. Noninvasive assessment of IDH mutational status in World Health Organization grade II and III astrocytomas using DWI and DSC-PWI combined with conventional MR imaging. AJNR Am J Neuroradiol. 2017;38:1138–44.
Lee S, Choi SH, Ryoo I, Yoon TJ, Kim TM, Lee SH, et al. Evaluation of the microenvironmental heterogeneity in high-grade gliomas with IDH1/2 gene mutation using histogram analysis of diffusion-weighted imaging and dynamic-susceptibility contrast perfusion imaging. J Neurooncol. 2015;121:141–50.
Tan WL, Huang WY, Yin B, Xiong J, Wu JS, Geng DY. Can diffusion tensor imaging noninvasively detect IDH1 gene mutations in astrogliomas? A retrospective study of 112 cases. AJNR Am J Neuroradiol. 2014;35:920–7.
Suzuki K, Kawai N, Ogawa T, Miyake K, Shinomiya A, Yamamoto Y, et al. Hypoxia and glucose metabolism assessed by FMISO and FDG PET for predicting IDH1 mutation and 1p/19q codeletion status in newly diagnosed malignant gliomas. EJNMMI Res. 2021;11:67.
Ogawa T, Kawai N, Miyake K, Shinomiya A, Yamamoto Y, Nishiyama Y, et al. Diagnostic value of PET/CT with (11)C-methionine (MET) and (18)F-fluorothymidine (FLT) in newly diagnosed glioma based on the 2016 WHO classification. EJNMMI Res. 2020;10:44.
Miyake K, Suzuki K, Ogawa T, Ogawa D, Hatakeyama T, Shinomiya A, et al. Multiple positron emission tomography tracers for use in the classification of gliomas according to the 2016 World Health Organization criteria. Neurooncol Adv. 2021;3:vdaa172.
Verger A, Stoffels G, Bauer EK, Lohmann P, Blau T, Fink GR, et al. Static and dynamic (18)F-FET PET for the characterization of gliomas defined by IDH and 1p/19q status. Eur J Nucl Med Mol Imaging. 2018;45:443–51.
Toyonaga T, Yamaguchi S, Hirata K, Kobayashi K, Manabe O, Watanabe S, et al. Hypoxic glucose metabolism in glioblastoma as a potential prognostic factor. Eur J Nucl Med Mol Imaging. 2017;44:611–9.
Bruehlmeier M, Roelcke U, Schubiger PA, Ametamey SM. Assessment of hypoxia and perfusion in human brain tumors using PET with 18F-fluoromisonidazole and 15O–H2O. J Nucl Med. 2004;45:1851–9.
Rasey JS, Koh WJ, Grierson JR, Grunbaum Z, Krohn KA. Radiolabelled fluoromisonidazole as an imaging agent for tumor hypoxia. Int J Radiat Oncol Biol Phys. 1989;17:985–91.
Ozturk K, Soylu E, Cayci Z. Correlation between dynamic susceptibility contrast perfusion MRI and genomic alterations in glioblastoma. Neuroradiology. 2021;63:1801–10.
Park YW, Han K, Ahn SS, Bae S, Choi YS, Chang JH, et al. Prediction of IDH1-mutation and 1p/19q-codeletion status using preoperative MR imaging phenotypes in lower grade gliomas. AJNR Am J Neuroradiol. 2018;39:37–42.
Funding
This study has received funding by The Uehara Memorial Foundation and the Kyoto University Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by Kyoto University Graduate School and Faculty of Medicine, Ethics Committee.
Informed consent
Written informed consent was obtained from all patients in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Wang, Y., Fushimi, Y., Arakawa, Y. et al. Evaluation of isocitrate dehydrogenase mutation in 2021 world health organization classification grade 3 and 4 glioma adult-type diffuse gliomas with 18F-fluoromisonidazole PET. Jpn J Radiol 41, 1255–1264 (2023). https://doi.org/10.1007/s11604-023-01450-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-023-01450-x