Abstract
Purpose
Knowledge of kidney stone composition can help in patient management; urine composition analysis and dual-energy CT are frequently used to assess stone type. We assessed if threshold-based stone segmentation and radiomics can determine the composition of kidney stones from single-energy, non-contrast abdomen–pelvis CT.
Methods
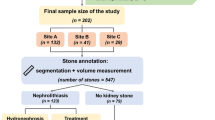
With IRB approval, we identified 218 consecutive patients (mean age 64 ± 13 years; male:female 138:80) with the presence of kidney stones on non-contrast, abdomen–pelvis CT and surgical or biochemical proof of their stone composition. CT examinations were performed on one of the seven multidetector-row scanners from four vendors (GE, Philips, Siemens, Toshiba). Deidentified CT images were processed with a radiomics prototype (Frontier, Siemens Healthineers) to segment the entire kidney volumes with an AI-based organ segmentation tool. We applied a threshold of 130 HU to isolate stones in the segmented kidneys and to estimate radiomics over the segmented stone volume. A coinvestigator verified kidney stone segmentation and adjusted the volume of interest to include the entire stone volume when necessary. We applied multiple logistic regression tests with precision recall plots to obtain area under the curve (AUC) using a built-in R statistical program.
Results
The threshold-based stone segmentation successfully isolated kidney stones (uric acid: n = 102 patients, calcium oxalate/phosphate: n = 116 patients) in all patients. Radiomics differentiated between calcium and uric acid stones with an AUC of 0.78 (p < 0.01, 95% CI 0.73–0.83), 0.79 sensitivity, and 0.90 specificity regardless of CT vendors (GE CT: AUC = 0.82, p < 0.01, 95% CI 0.740–0896; Siemens CT: AUC = 0.77, 95% CI 0.700–0.846, p < 0.01).
Conclusion
Automated threshold-based stone segmentation and radiomics can differentiate between calcium oxalate/phosphate and urate stones from non-contrast, single-energy abdomen CT.


Similar content being viewed by others
References
Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol. 2010;12(2–3):e86-96.
Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 2003;63(5):1817–23.
Scales CD Jr, Smith AC, Hanley JM, Saigal CS. Prevalence of kidney stones in the United States. Eur Urol. 2012;62(1):160–5.
Grases F, Costa-Bauzá A, García-Ferragut L. Biopathological crystallization: a general view about the mechanisms of renal stone formation. Adv Colloid Interface Sci. 1998;74:169–94.
Anderson RA. A complementary approach to urolithiasis prevention. World J Urol. 2002;20(5):294–301.
Cloutier J, Villa L, Traxer O, Daudon M. Kidney stone analysis: “give me your stone, i will tell you who you are!” World J Urol. 2015;33(2):157–69.
Graff J, Diederichs W, Schulze H. Long-term followup in 1003 extracorporeal shock wave lithotripsy patients. J Urol. 1988;140(3):479–83.
Bultitude M, Smith D, Thomas K. Contemporary management of stone disease: the new EAU urolithiasis guidelines for 2015. Eur Urol. 2016;69(3):483–4.
Tamm EP, Le O, Liu X, Layman RR, Cody DD, Bhosale PR. “How to” incorporate dual-energy imaging into a high volume abdominal imaging practice. Abdom Radio. 2017;42(3):688–701.
Abraham A, Kavoussi NL, Sui W, Bejan C, Capra JA, Hsi R. Machine learning prediction of kidney stone composition using electronic health record-derived features. J Endourol. 2022;36(2):243–50.
Tang L, Li W, Zeng X, Wang R, Yang X, Luo G, Chen Q, Wang L, Song B. Value of artificial intelligence model based on unenhanced computed tomography of urinary tract for preoperative prediction of calcium oxalate monohydrate stones in vivo. Ann Transl Med. 2021;9(14).
Onal EG, Tekgul H. Assessing kidney stone composition using smartphone microscopy and deep neural networks. BJUI Compass. 2022.
Nestler T, Haneder S, Hokamp NG. Modern imaging techniques in urinary stone disease. Curr Opin Urol. 2019;29(2):81–8.
Wels MG, Lades F, Muehlberg A, Suehling M. General purpose radiomics for multi-modal clinical research. In: Medical imaging 2019: computer-aided diagnosis; 2019. Vol. 10950: p. 1095046. International Society for Optics and Photonics.
Hidas G, Eliahou R, Duvdevani M, Coulon P, Lemaitre L, Gofrit ON, Pode D, Sosna J. Determination of renal stone composition with dual-energy CT: in vivo analysis and comparison with x-ray diffraction. Radiology. 2010;257(2):394–401.
Demehri S, Kalra MK, Rybicki FJ, Steigner ML, Lang MJ, Houseman EA, Curhan GC, Silverman SG. Quantification of urinary stone volume: attenuation threshold–based CT method—a technical note. Radiology. 2011;258(3):915–22.
Homayounieh F, Khera RD, Bizzo BC, Ebrahimian S, Primak A, Schmidt B, Saini S, Kalra MK. Prediction of burden and management of renal calculi from whole kidney radiomics: a multicenter study. Abdom Radiol. 2021;46(5):2097–106.
De Perrot T, Hofmeister J, Burgermeister S, Martin SP, Feutry G, Klein J, Montet X. Differentiating kidney stones from phleboliths in unenhanced low-dose computed tomography using radiomics and machine learning. Eur Radiol. 2019;29(9):4776–82.
Jendeberg J, Thunberg P, Popiolek M, Lidén M. Single-energy CT predicts uric acid stones with accuracy comparable to dual-energy CT—prospective validation of a quantitative method. Eur Radiol. 2021;31(8):5980–89.
Zhang GM, Sun H, Shi B, Xu M, Xue HD, Jin ZY. Uric acid versus non-uric acid urinary stones: differentiation with single energy CT texture analysis. Clin Radiol. 2018;73(9):792–9.
Tang X, Pang T, Yan WF, Qian WL, Gong YL, Yang ZG. The prognostic value of radiomics features extracted from computed tomography in patients with localized clear cell renal cell carcinoma after nephrectomy. Front Oncol. 2021;11:85.
Black KM, Law H, Aldoukhi AH, Roberts WW, Deng J, Ghani KR. Deep learning computer vision algorithm for detecting kidney stone composition: towards an automated future. Eur Urol Suppl. 2019;18(1):e853–4.
Lidén M. A new method for predicting uric acid composition in urinary stones using routine single-energy CT. Urolithiasis. 2018;46(4):325–32.
Cui X, Che F, Wang N, Liu X, Zhu Y, Zhao Y, Bi J, Li Z, Zhang G. Preoperative prediction of infection stones using radiomics features from computed tomography. IEEE Access. 2019;7:122675–83.
Robins M, Solomon J, Hoye J, Abadi E, Marin D, Samei E. Systematic analysis of bias and variability of texture measurements in computed tomography. J Med Imaging. 2019;6(3):033503.21.
Spek A, Strittmatter F, Graser A, Kufer P, Stief C, Staehler M. Dual energy can accurately differentiate uric acid-containing urinary calculi from calcium stones. World J Urol. 2016;34(9):1297–302.
Funding
This work has not received any findings. A study coinvestigator received research grants for unrelated work (MKK—Siemens Healthineers, Riverain Tech, Coreline Inc.). Another study coauthor (AP) is an employee of Siemens Healthineers. No other co-authors have financial disclosures.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kaviani, P., Primak, A., Bizzo, B. et al. Performance of threshold-based stone segmentation and radiomics for determining the composition of kidney stones from single-energy CT. Jpn J Radiol 41, 194–200 (2023). https://doi.org/10.1007/s11604-022-01349-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-022-01349-z