Abstract
Purpose
This study aimed to use dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) to evaluate early treatment response in vestibular schwannoma (VS) patients after radiosurgery.
Methods
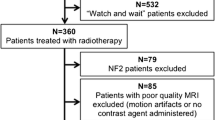
Twenty-four VS patients who underwent gamma knife radiosurgery were prospectively followed up for at least four years. DCE-MRI sequences, in addition to standard MRI protocol, were obtained prior to radiosurgery, at 3 and 6 months. Conventionally, treatment responses based on tumor volume changes were classified as regression or stable (RS), transient tumor enlargement (TTE), and continuous tumor enlargement (CTE). DCE-MRI parameters, such as Ktrans, Kep and Ve, were compared according to follow-up periods and between groups. The diagnostic performance was tested using receiver operating characteristic (ROC) curves.
Results
Changes in tumor volume were as follows at the last 48 months of follow-up: RS in 11 patients (45.8%), TTE in 10 patients (41.7%), and CTE in three patients (12.5%). The median time required to distinguish TTE from CTE using conventional MRI was 12 months (range 9–18). The Ktrans and Ve were significantly decreased in patients with RS and TTE at 3 and 6 months, but did not differ significantly in patients with CTE. There were no significant differences in Ktrans and Ve between patients with RS and TTE at 3 and 6 months. Both Ktrans and Ve demonstrated high diagnostic performance in evaluating early treatment response to radiosurgery in patients with VS.
Conclusion
DCE-MRI may aid in the monitoring and early prediction of treatment response in patients with VS following radiosurgery.






Similar content being viewed by others
Abbreviations
- VS:
-
Vestibular schwannoma
- MRI:
-
Magnetic resonance imaging
- DCE-MRI:
-
Dynamic contrast-enhanced magnetic resonance imaging
- K trans :
-
Volumetric transfer constant
- V e :
-
Fractional volume of extracellular extravascular space
- K ep :
-
Rate constant
- RS:
-
Regression or stable
- TTE:
-
Transient tumor enlargement
- CTE:
-
Continuous tumor enlargement
References
Mohan S, Hoeffner E, Bigelow DC, Loevner LA. Applications of magnetic resonance imaging in adult temporal bone disorders. Magn Reson Imaging Clin N Am. 2012;20(3):545–72. https://doi.org/10.1016/j.mric.2012.06.001.
Battista RA. Gamma knife radiosurgery for vestibular schwannoma. Otolaryngol Clin N Am. 2009;42(4):635–54. https://doi.org/10.1016/j.otc.2009.04.009.
Meijer OW, Weijmans EJ, Knol DL, Slotman BJ, Barkhof F, Vandertop WP, Castelijns JA. Tumor-volume changes after radiosurgery for vestibular schwannoma: implications for follow-up MR imaging protocol. AJNR Am J Neuroradiol. 2008;29(5):906–10. https://doi.org/10.3174/ajnr.A0969.
Nakamura H, Jokura H, Takahashi K, Boku N, Akabane A, Yoshimoto T. Serial follow-up MR imaging after gamma knife radiosurgery for vestibular schwannoma. AJNR Am J Neuroradiol. 2000;21(8):1540–6.
Hasegawa T, Kida Y, Kobayashi T, Yoshimoto M, Mori Y, Yoshida J. Long-term outcomes in patients with vestibular schwannomas treated using gamma knife surgery: 10-year follow up. J Neurosurg. 2005;102(1):10–6. https://doi.org/10.3171/jns.2005.102.1.0010.
Hasegawa T, Kida Y, Yoshimoto M, Koike J, Goto K. Evaluation of tumor expansion after stereotactic radiosurgery in patients harboring vestibular schwannomas. Neurosurgery. 2006;58(6):1119–28. https://doi.org/10.1227/01.Neu.0000215947.35646.Dd (discussion 1119–1128).
Okunaga T, Matsuo T, Hayashi N, Hayashi Y, Shabani HK, Kaminogo M, Ochi M, Nagata I. Linear accelerator radiosurgery for vestibular schwannoma: measuring tumor volume changes on serial three-dimensional spoiled gradient-echo magnetic resonance images. J Neurosurg. 2005;103(1):53–8. https://doi.org/10.3171/jns.2005.103.1.0053.
Hayhurst C, Zadeh G. Tumor pseudoprogression following radiosurgery for vestibular schwannoma. Neuro Oncol. 2012;14(1):87–92. https://doi.org/10.1093/neuonc/nor171.
Nagano O, Higuchi Y, Serizawa T, Ono J, Matsuda S, Yamakami I, Saeki N. Transient expansion of vestibular schwannoma following stereotactic radiosurgery. J Neurosurg. 2008;109(5):811–6. https://doi.org/10.3171/jns/2008/109/11/0811.
Abramson RG, Arlinghaus LR, Dula AN, Quarles CC, Stokes AM, Weis JA, Whisenant JG, Chekmenev EY, Zhukov I, Williams JM, Yankeelov TE. MR imaging biomarkers in oncology clinical trials. Magn Reson Imaging Clin N Am. 2016;24(1):11–29. https://doi.org/10.1016/j.mric.2015.08.002.
García-Figueiras R, Padhani AR, Baleato-González S. Therapy monitoring with functional and molecular MR imaging. Magn Reson Imaging Clin N Am. 2016;24(1):261–88. https://doi.org/10.1016/j.mric.2015.08.003.
Lin YC, Wang CC, Wai YY, Wan YL, Ng SH, Chen YL, Liu HL, Wang JJ. Significant temporal evolution of diffusion anisotropy for evaluating early response to radiosurgery in patients with vestibular schwannoma: findings from functional diffusion maps. AJNR Am J Neuroradiol. 2010;31(2):269–74. https://doi.org/10.3174/ajnr.A1799.
Chawla S, Kim S, Loevner LA, Hwang WT, Weinstein G, Chalian A, Quon H, Poptani H. Prediction of disease-free survival in patients with squamous cell carcinomas of the head and neck using dynamic contrast-enhanced MR imaging. AJNR Am J Neuroradiol. 2011;32(4):778–84. https://doi.org/10.3174/ajnr.A2376.
Kim S, Loevner LA, Quon H, Kilger A, Sherman E, Weinstein G, Chalian A, Poptani H. Prediction of response to chemoradiation therapy in squamous cell carcinomas of the head and neck using dynamic contrast-enhanced MR imaging. AJNR Am J Neuroradiol. 2010;31(2):262–8. https://doi.org/10.3174/ajnr.A1817.
Padhani AR, Hayes C, Assersohn L, Powles T, Makris A, Suckling J, Leach MO, Husband JE. Prediction of clinicopathologic response of breast cancer to primary chemotherapy at contrast-enhanced MR imaging: initial clinical results. Radiology. 2006;239(2):361–74. https://doi.org/10.1148/radiol.2392021099.
Johansen R, Jensen LR, Rydland J, Goa PE, Kvistad KA, Bathen TF, Axelson DE, Lundgren S, Gribbestad IS. Predicting survival and early clinical response to primary chemotherapy for patients with locally advanced breast cancer using DCE-MRI. J Magn Reson Imaging. 2009;29(6):1300–7. https://doi.org/10.1002/jmri.21778.
Petrillo A, Fusco R, Petrillo M, Granata V, Bianco F, Di Marzo M, Delrio P, Tatangelo F, Botti G, Pecori B, Avallone A. DCE-MRI time–intensity curve visual inspection to assess pathological response after neoadjuvant therapy in locally advanced rectal cancer. Jpn J Radiol. 2018;36(10):611–21. https://doi.org/10.1007/s11604-018-0760-1.
Kato E, Mori N, Mugikura S, Sato S, Ishida T, Takase K. Value of ultrafast and standard dynamic contrast-enhanced magnetic resonance imaging in the evaluation of the presence and extension of residual disease after neoadjuvant chemotherapy in breast cancer. Jpn J Radiol. 2021;39(8):791–801. https://doi.org/10.1007/s11604-021-01110-y.
Griffith B, Jain R. Perfusion imaging in neuro-oncology: basic techniques and clinical applications. Radiol Clin N Am. 2015;53(3):497–511. https://doi.org/10.1016/j.rcl.2015.01.004.
Lacerda S, Law M. Magnetic resonance perfusion and permeability imaging in brain tumors. Neuroimaging Clin N Am. 2009;19(4):527–57. https://doi.org/10.1016/j.nic.2009.08.007.
Paldino MJ, Barboriak DP. Fundamentals of quantitative dynamic contrast-enhanced MR imaging. Magn Reson Imaging Clin N Am. 2009;17(2):277–89. https://doi.org/10.1016/j.mric.2009.01.007.
Salem A, O’Connor JPB. Assessment of tumor angiogenesis: dynamic contrast-enhanced MR imaging and beyond. Magn Reson Imaging Clin N Am. 2016;24(1):45–56. https://doi.org/10.1016/j.mric.2015.08.010.
Türkbey B, Thomasson D, Pang Y, Bernardo M, Choyke PL. The role of dynamic contrast-enhanced MRI in cancer diagnosis and treatment. Diagn Interv Radiol. 2010;16(3):186–92. https://doi.org/10.4261/1305-3825.Dir.2537-08.1.
Gardner G, Robertson JH. Hearing preservation in unilateral acoustic neuroma surgery. Ann Otol Rhinol Laryngol. 1988;97(1):55–66. https://doi.org/10.1177/000348948809700110.
Almeida-Freitas DB, Pinho MC, Otaduy MC, Braga HF, Meira-Freitas D, da Costa LC. Assessment of irradiated brain metastases using dynamic contrast-enhanced magnetic resonance imaging. Neuroradiology. 2014;56(6):437–43. https://doi.org/10.1007/s00234-014-1344-0.
Li KL, Djoukhadar I, Zhu X, Zhao S, Lloyd S, McCabe M, McBain C, Evans DG, Jackson A. Vascular biomarkers derived from dynamic contrast-enhanced MRI predict response of vestibular schwannoma to antiangiogenic therapy in type 2 neurofibromatosis. Neuro Oncol. 2016;18(2):275–82. https://doi.org/10.1093/neuonc/nov168.
Li SP, Makris A, Beresford MJ, Taylor NJ, Ah-See ML, Stirling JJ, d’Arcy JA, Collins DJ, Kozarski R, Padhani AR. Use of dynamic contrast-enhanced MR imaging to predict survival in patients with primary breast cancer undergoing neoadjuvant chemotherapy. Radiology. 2011;260(1):68–78. https://doi.org/10.1148/radiol.11102493.
Hötker AM, Tarlinton L, Mazaheri Y, Woo KM, Gönen M, Saltz LB, Goodman KA, Garcia-Aguilar J, Gollub MJ. Multiparametric MRI in the assessment of response of rectal cancer to neoadjuvant chemoradiotherapy: a comparison of morphological, volumetric and functional MRI parameters. Eur Radiol. 2016;26(12):4303–12. https://doi.org/10.1007/s00330-016-4283-9.
Martens MH, Subhani S, Heijnen LA, Lambregts DM, Buijsen J, Maas M, Riedl RG, Jeukens CR, Beets GL, Kluza E, Beets-Tan RG. Can perfusion MRI predict response to preoperative treatment in rectal cancer? Radiother Oncol. 2015;114(2):218–23. https://doi.org/10.1016/j.radonc.2014.11.044.
Sahani DV, Jiang T, Hayano K, Duda DG, Catalano OA, Ancukiewicz M, Jain RK, Zhu AX. Magnetic resonance imaging biomarkers in hepatocellular carcinoma: association with response and circulating biomarkers after sunitinib therapy. J Hematol Oncol. 2013;6:51. https://doi.org/10.1186/1756-8722-6-51.
Sun NN, Liu C, Ge XL, Wang J. Dynamic contrast-enhanced MRI for advanced esophageal cancer response assessment after concurrent chemoradiotherapy. Diagn Interv Radiol. 2018;24(4):195–202. https://doi.org/10.5152/dir.2018.17369.
Peng SL, Chen CF, Liu HL, Lui CC, Huang YJ, Lee TH, Chang CC, Wang FN. Analysis of parametric histogram from dynamic contrast-enhanced MRI: application in evaluating brain tumor response to radiotherapy. NMR Biomed. 2013;26(4):443–50. https://doi.org/10.1002/nbm.2882.
Jakubovic R, Sahgal A, Soliman H, Milwid R, Zhang L, Eilaghi A, Aviv RI. Magnetic resonance imaging-based tumour perfusion parameters are biomarkers predicting response after radiation to brain metastases. Clin Oncol (R Coll Radiol). 2014;26(11):704–12. https://doi.org/10.1016/j.clon.2014.06.010.
Lu F, Li YQ, Aubert I, Wong CS. Endothelial cells regulate p53-dependent apoptosis of neural progenitors after irradiation. Cell Death Dis. 2012;3(6): e324. https://doi.org/10.1038/cddis.2012.59.
Folkman J, Camphausen K. Cancer. What does radiotherapy do to endothelial cells? Science. 2001;293(5528):227–8. https://doi.org/10.1126/science.1062892.
Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, Birrer MJ, Szabo E, Zon LI, Kyriakis JM, Haimovitz-Friedman A, Fuks Z, Kolesnick RN. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380(6569):75–9. https://doi.org/10.1038/380075a0.
Nordal RA, Wong CS. Molecular targets in radiation-induced blood-brain barrier disruption. Int J Radiat Oncol Biol Phys. 2005;62(1):279–87. https://doi.org/10.1016/j.ijrobp.2005.01.039.
Hirato M, Inoue H, Nakamura M, Ohye C, Hirato J, Shibazaki T, Andou Y. Gamma knife radiosurgery for acoustic schwannoma: early effects and preservation of hearing. Neurol Med Chir (Tokyo). 1995;35(10):737–41. https://doi.org/10.2176/nmc.35.737.
Kobayashi T, Tanaka T, Kida Y. The early effects of gamma knife on 40 cases of acoustic neurinoma. Acta Neurochir Suppl. 1994;62:93–7. https://doi.org/10.1007/978-3-7091-9371-6_19.
Xu QG, Xian JF. Role of quantitative magnetic resonance imaging parameters in the evaluation of treatment response in malignant tumors. Chin Med J (Engl). 2015;128(8):1128–33. https://doi.org/10.4103/0366-6999.155127.
Jain RK. Transport of molecules in the tumor interstitium: a review. Cancer Res. 1987;47(12):3039–51.
Tong T, Sun Y, Gollub MJ, Peng W, Cai S, Zhang Z, Gu Y. Dynamic contrast-enhanced MRI: use in predicting pathological complete response to neoadjuvant chemoradiation in locally advanced rectal cancer. J Magn Reson Imaging. 2015;42(3):673–80. https://doi.org/10.1002/jmri.24835.
Guo J, Reddick WE, Glass JO, Ji Q, Billups CA, Wu J, Hoffer FA, Kaste SC, Jenkins JJ, Ortega Flores XC, Quintana J, Villarroel M, Daw NC. Dynamic contrast-enhanced magnetic resonance imaging as a prognostic factor in predicting event-free and overall survival in pediatric patients with osteosarcoma. Cancer. 2012;118(15):3776–85. https://doi.org/10.1002/cncr.26701.
Lim JS, Kim D, Baek SE, Myoung S, Choi J, Shin SJ, Kim MJ, Kim NK, Suh J, Kim KW, Keum KC. Perfusion MRI for the prediction of treatment response after preoperative chemoradiotherapy in locally advanced rectal cancer. Eur Radiol. 2012;22(8):1693–700. https://doi.org/10.1007/s00330-012-2416-3.
Chikui T, Kawano S, Kawazu T, Hatakenaka M, Koga S, Ohga M, Matsuo Y, Sunami S, Sugiura T, Shioyama Y, Obara M, Yoshiura K. Prediction and monitoring of the response to chemoradiotherapy in oral squamous cell carcinomas using a pharmacokinetic analysis based on the dynamic contrast-enhanced MR imaging findings. Eur Radiol. 2011;21(8):1699–708. https://doi.org/10.1007/s00330-011-2102-x.
Wheeler JM, Warren BF, Jones AC, Mortensen NJ. Preoperative radiotherapy for rectal cancer: implications for surgeons, pathologists and radiologists. Br J Surg. 1999;86(9):1108–20. https://doi.org/10.1046/j.1365-2168.1999.01209.x.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: HÖ, MY, NE, AYÖ; methodology: HÖ, MY, NE, GK, ÖHE, AYÖ; formal analysis and investigation: HÖ, MY, NE; writer: HÖ; writing—review and editing: MY, ÖHE, GK, AYÖ. Supervision: ÖHE, GK, AYÖ.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study approved by the institutional ethics committee (336/2016).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Özer, H., Yazol, M., Erdoğan, N. et al. Dynamic contrast-enhanced magnetic resonance imaging for evaluating early response to radiosurgery in patients with vestibular schwannoma. Jpn J Radiol 40, 678–688 (2022). https://doi.org/10.1007/s11604-021-01245-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-021-01245-y