Abstract
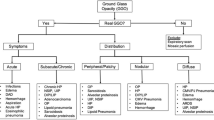
Non-neoplastic lesions of central airways are uncommon entities with different etiologies, with either focal or diffuse involvement of the tracheobronchial tree. Clinical symptoms of non-neoplastic tracheobronchial diseases are non-specific, and diagnosis is difficult, especially in the early stages. Three-dimensional computed tomography (3D-CT) is an evaluable tool as it allows to assess and characterize tracheobronchial wall lesions and meanwhile it enables the evaluation of airways surrounding structures. Multiplanar reconstructions (MPR), minimum intensity projections (MinIP), and 3D Volume Rendering (VR) (in particular, virtual bronchoscopy) also provide information on the site and of the length of airway alterations. This review will be discussed about (1) primary airway disorders, such as relapsing polychondritis, tracheobronchophathia osteochondroplastica, and tracheobronchomegaly, (2) airway diseases, related to granulomatosis with polyangiitis, Chron's disease, Behcet's disease, sarcoidosis, amyloidosis, infections, intubation and transplantation, (3) tracheobronchial malacia, and (4) acute tracheobronchial injury. 3D-CT findings, especially with MPR and 3D VR reconstructions, allows us to evaluate tracheobronchial disease morphologically in detail.










Similar content being viewed by others
References
Airways and Esophagus Nonneoplastic Lesions of the Tracheobron-chial Wall: Radiologic Findings with Bron-choscopic Correlation 1.
Lawrence DA, Branson B, Oliva I, Rubinowitz A. The wonderful world of the windpipe: a review of central airway anatomy and pathology. Can Assoc Radiol J. 2015;66:30–43.
Shepard JAO, Flores EJ, Abbott GF. Imaging of the trachea. Ann Cardiothor Surg. 2018;7:197–209.
Obusez EC, Jamjoom L, Kirsch J, Gildea T, Mohammed TL. Computed tomography correlation of airway disease with bronchoscopy: part i-nonneoplastic large airway diseases. Curr Probl Diagn Radiol. 2014;43:268–77.
Chung JH, Kanne JP, Gilman MD. CT of diffuse tracheal diseases. Am J Roentgenol. 2011;196:W240–6.
Morshed K, et al. Evaluation of tracheal stenosis: Comparison between computed tomography virtual tracheobronchoscopy with multiplanar reformatting, flexible tracheofiberoscopy and intra-operative findings. Eur Arch Otorhinolaryngol. 2011;268:591–7.
White BD, Kong A, Khoo E, Southcott AM. Computed tomography diagnosis of tracheobronchopathia osteochondroplastica. Aust Radiol. 2005;49:319–21.
Kafili D, Sampson T, Tolhurst S. Difficult intubation in an asymptomatic patient with tracheobronchopathia osteochondroplastica. Respirol Case Rep. 2020;8:e00526.
Thakur A, et al. Atypical presentation of tracheobronchopathia osteochondroplastica: is chronic inflammation a perpetrator? Med Princ Pract. 2013;22:503–5.
Luo T, Zhou H, Meng J. Clinical characteristics of tracheobronchopathia osteochondroplastica. Respir Care. 2019;64:196–200.
Kwon W, Lee SM, Bang S. Costoclavicular block for shoulder surgery in a patient with tracheobronchopathia osteochondroplastica and COPD. J Clin Anesth. 2019;55:13–4.
Ulasli SS, Kupeli E. Tracheobronchopathia osteochondroplastica: A review of the literature. Clin Respir J. 2015;9:386–91.
Woźniak, M. S., Buchwald, J., Stockdale, I., Doniec, Z. Tracheobronchopathia osteochondroplastica-a 61-year-old female with middle lobe syndrome Case report (2017). https://doi.org/10.5603/ARM.2017.0027 Praca oryginalna 158 Case Report www.journals.viamedica.pl
Zack JR, Rozenshtein A. Tracheobronchopathia Osteochondroplastica: report of Three Cases. J Comput Assist Tomogr. 2002;26:33–6.
Restrepo S, et al. Tracheobronchopathia osteochondroplastica: helical CT findings in 4 cases. J Thorac Imaging. 2004;19(2):112–6.
Jindal S, Nath A, Neyaz Z, Jaiswal S. Tracheobronchopathia osteochondroplastica—a rare or an overlooked entity? J Radiol Case Rep. 2013;7:16–25.
Borgia F, Giuffrida R, Guarneri F, Cannavò SP. Relapsing polychondritis: an updated review. Biomedicines. 2018;6:84.
Dion J, et al. Relapsing polychondritis can be characterized by three different clinical phenotypes: analysis of a recent series of 142 patients. Arthrit Rheumatol. 2016;68:2992–3001.
Rednic S, et al. Relapsing polychondritis: State of the art on clinical practice guidelines. RMD open. 2018;4:e000788.
Maciążek-Chyra B, et al. Relapsing polychondritis—analysis of symptoms and criteria. Reumatologia. 2019;57:8–18.
Marchiori E, Hochhegger B, Zanetti G. Thickening of the tracheal wall. Jornal Brasileiro de Pneumologia. 2017;43:251–251. https://doi.org/10.1590/S1806-37562017000000074.
de Montmollin N, Dusser D, Lorut C, Dion J, Costedoat-Chalumeau N, Mouthon L, Puéchal X. Tracheobronchial involvement of relapsing polychondritis. Autoimmun Rev. 2019;18:102353.
Kuwal A, et al. An atypical case of mounier-kuhn syndrome: time to change the diagnostic approach? J Bronchol Intervent Pulmonol. 2017;24:84–7.
Girit S, Senol E, Cag Y, Karatas O, Baysal T. Ehlers-danlos syndrome type IVB and tracheobronchomegaly. J Bronchol Intervent Pulmonol. 2018;25:70–2.
Menon B, Aggarwal B, Iqbal A. Mounier–Kuhn syndrome: report of 8 cases of tracheobronchomegaly with associated complications. South Med J. 2008;101:83–7.
Unlu EN, et al. An unusual cause of recurrent spontaneous pneumothorax: the Mounier–Kuhn syndrome. Am J Emerg Med. 2016;34(122):e1-122.e2.
Kumar S, Mittal AK. Mounier-Kuhn syndrome (MKS)—pathognomonic findings. J Clin Diagn Res. 2014;8:RJ01–2.
Naciri S, Zahraoui R, Soualhi M, Bourkadi J-E. An unusual cause of spontaneous pneumomediastinum: the mounier–kuhn syndrome. Case Rep Pulmonol. 2019;2019:1–5.
Krustins E. Mounier–Kuhn syndrome: a systematic analysis of 128 cases published within last 25 years. Clin Respir J. 2016;10:3–10.
No H-J, Lee J-M, Won D, Kang P, Choi S. Airway management of a patient incidentally diagnosed with Mounier-Kuhn syndrome during general anesthesia. J Dental Anesth Pain Med. 2019;19:301.
Govindaraj V, MohantyMohapatra M, Nagamalli Kumar B, Narayanasami S. Tracheobronchomegaly as a cause of bronchiectasis in an adult. Case Rep Pulmonol. 2016;2016:1–4.
Jennette JC. Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin Exp Nephrol. 2013;17:603–6.
Allen SD, Harvey CJ. Imaging of Wegener’s granulomatosis. Br J Radiol. 2007;80:757–65.
Ananthakrishnan L, Sharma N, Kanne JP. Wegener’s granulomatosis in the chest: high-resolution CT findings. Am J Roentgenol. 2009;192:676–82.
Girard C, et al. Tracheobronchial stenoses in granulomatosis with polyangiitis (Wegener’s) a report on 26 cases. Medicine. 2015. https://doi.org/10.1097/MD.0000000000001088.
Martinez Del Pero M, Rasmussen N, Chaudhry A, Jani P, Jayne D. Structured clinical assessment of the ear, nose and throat in patients with granulomatosis with polyangiitis (Wegener’s). Eur Arch Otorhinolaryngol. 2012. https://doi.org/10.1007/s00405-012-2110-8.
Solans-Laqué R, et al. Clinical features and therapeutic management of subglottic stenosis in patients with Wegener’s granulomatosis. Lupus. 2008;17:832–6.
Zycinska K, et al. Subglottic and tracheal stenosis due to Wegener’s granulomatosis. In: Advances in experimental medicine and biology vol. 755. Springer Science and Business Media, LLC, 221–224 (2013)
Puchalski J, Musani AI. Tracheobronchial stenosis. Causes and advances in management. Clin Chest Med. 2013;34:557–67.
Sivasothy J, Flower PDR, Lockwood CDR. Tracheal involvement in Wegener’s granulomatosis: evaluation using spiral CT. Clin Radiol. 1998;53:809–15.
Grenier PA, Beigelman-Aubry C, Brillet PY. Nonneoplastic tracheal and bronchial stenoses. Radiol Clin North Am. 2009;47:243–60.
Lu X, et al. Bronchoscopic diagnosis and treatment of primary tracheobronchial amyloidosis: a retrospective analysis from China. BioMed Res Int. 2017. https://doi.org/10.1155/2017/3425812.
Cozzi D, et al. Radiological patterns of lung involvement in inflammatory bowel disease. Gastroenterol Res Pract. 2018. https://doi.org/10.1155/2018/5697846.
Kuzniar T, et al. Severe tracheobronchial stenosis in a patient with Crohn’s disease. Eur Respir J. 2000;15(1):209–12.
Plataki M, et al. Severe airway stenosis associated with Crohn’s disease: case report. BMC Pulm Med. 2006;6:1–5.
Davatchi F, et al. Behcet’s disease: epidemiology, clinical manifestations, and diagnosis. Expert Rev Clin Immunol. 2017;13:57–65.
Tran C, Daccord C, Nicod LP, Lazor R, et al. Cystic lung disease in genetic syndromes with deficient tumor suppressor gene function. Respiration. 2018;96:12–28.
Hajialilo M, Nasemieh M, Khabbazi A. Supraglottic stenosis in a case of Behcet’s disease. Scand J Rheumatol. 2018;47:164–5.
Gross M, Ben-Chetrit E. Laryngeal involvement in Behcet_s disease-a challenge for treatment. Clin Rheumatol. 2010. https://doi.org/10.1007/s10067-010-1501-8.
Khoor A, Colby TV. Amyloidosis of the lung. Arch Pathol Lab Med. 2017;141(2):247–54. https://doi.org/10.5858/arpa.2016-0102-RA.
Takumi K, et al. Amyloidosis in the head and neck: CT findings with clinicopathological correlation. Eur J Radiol. 2020;128:109034.
Czeyda-Pommersheim F, Hwang M, Chen SS, Strollo D, Fuhrman C, Bhalla S. Amyloidosis: modern cross-sectional imaging. Radiographics. 2015;35:1381–92. https://doi.org/10.1148/rg.2015140179.
Hosur B, Gupta V, Gupta K, Bakshi J. Radiological appearance of primary laryngotracheal amyloidosis. Lung India. 2019;36:441–4.
Costabel U, Hunninghake GW. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J 1999. 14(4):735–7. https://doi.org/10.1034/j.1399-3003.1999.14d02.x.
Shroff GS, et al. Pathology of the trachea and central bronchi. Semin Ultras CT MRI. 2016;37:177–89.
Darr A, Mohamed S, Eaton D, Kalkat M, Darr A. Tracheo-oesophageal fistula in a patient with chronic sarcoidosis. Ann R Coll Surg Engl. 2015. https://doi.org/10.1308/003588415X14181254790446.
Ding XM, et al. Impact of tracheal Mucosa involvement on clinical characteristics of sarcoidosis. South Med J. 2011;104:315–8.
Marchioni A, et al. Incidence, etiology, and clinicopathologic features of endobronchial benign lesions. J of Bronchol Intervent Pulmonol. 2018;25:118–24.
Cozzi D, et al. Atypical HRCT manifestations of pulmonary sarcoidosis. La Radiologia Medica. 2017;123:174–84.
Smati B, et al. Tuberculosis of the Trachea. Ann Thorac Surg. 2006;82:1900–1.
Kashyap S, Solanki A. Challenges in endobronchial tuberculosis: from diagnosis to management. Pulm Med. 2014. https://doi.org/10.1155/2014/594806.
Sangal V, Hoskisson PA. Evolution, epidemiology and diversity of corynebacterium diphtheriae: new perspectives on an old foe. Infect Genet Evol. 2016;43:364–70.
Adler NR, Mahony A, Friedman ND. Diphtheria: forgotten, but not gone. Intern Med J. 2013;43:206–10.
Antoniou T, et al. Secondary tracheal distortion in an adult patient after therapy for diphtheria at childhood. J Card Surg. 2020;35:1115–8.
Carretta A, et al. Preoperative assessment in patients with postintubation tracheal stenosis Rigid and flexible bronchoscopy versus spiral CT scan with multiplanar reconstructions. Surg Endosc. 2006. https://doi.org/10.1007/s00464-005-0475-0.
Bogush N, Eberlein M, Sanchez PG, Reed RM, Reed RM. Use of expiratory CT images in the diagnosis and localisation of airway complications following lung transplantation. Case Rep. 2015. https://doi.org/10.1136/bcr-2015.
Machuzak M, Santacruz JF, Gildea T, Murthy SC. Airway complications after lung transplantation. Thorac Cardiovasc Surg. 2015;25:55–75.
Santacruz JF, Mehta AC. Airway complications and management after lung transplantation: Ischemia, dehiscence, and stenosis. Proc Am Thorac Soc. 2009;6:79–93.
Wallis C, et al. ERS statement on tracheomalacia and bronchomalacia in children. Eur Respir J . 2019. https://doi.org/10.1183/1399300300382.
Kugler C, Stanzel F. Tracheomalacia. Thorac Cardiovasc Surg. 2014;24:51–8.
Wright CD. Tracheobronchomalacia and expiratory collapse of central airways. Thorac Cardiovasc Surg. 2018;28:163–6.
Cohen SL, et al. Ultralow dose dynamic expiratory computed tomography for evaluation of tracheomalacia. J Comput Assist Tomogr. 2019;43:307–11.
Godoy MC, Saldana DA, Rao PP, Vlahos I, Naidich DP, Benveniste MF, Ost D. Multidetector CT evaluation of airway stents: what the radiologist should know. Radiographics. 2014;34:1793–806. https://doi.org/10.1148/rg.347130063.
Welter S. Repair of tracheobronchial injuries. Thorac Cardiovasc Surg. 2014;24:41–50.
Kovacs G, Sowers N. Airway management in trauma. Emerg Med Clin North Am. 2018;36:61–84.
Prokakis C. et al. Airway trauma: a review on epidemiology, mechanisms of injury, diagnosis and treatment. (2014). https://doi.org/10.1186/1749-8090-9-117. http://www.cardiothoracicsurgery.org/content/9/1/117
Scaglione M, et al. Acute tracheobronchial injuries: Impact of imaging on diagnosis and management implications. Eur J Radiol. 2006;59:336–43.
Bagga B, Kumar A, Chahal A, Gamanagatti S, Kumar S. Traumatic airway injuries: role of imaging. Curr Probl Diagn Radiol. 2020;49:48–53.
Funding
The Authors declare that they do not receive any funding for the publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The Authors declare that they have no conflict of interest related to the publication of this article.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Human and animal rights
This article does not contain any studies with animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Moroni, C., Bindi, A., Cavigli, E. et al. CT findings of non-neoplastic central airways diseases. Jpn J Radiol 40, 107–119 (2022). https://doi.org/10.1007/s11604-021-01190-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-021-01190-w