Abstract
Purpose
To evaluate the role of susceptibility-weighted imaging (SWI) in patients with idiopathic intracranial hypertension (IIH).
Materials and methods
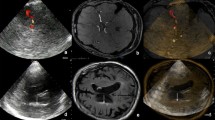
A prospective study was done on 55 patients with IIH who underwent SWI of the brain. The images were evaluated by two independent readers for cerebral microbleeds (CMBs) and the interobserver agreement between both readers was calculated. The graphic rating scale (GRS) for headache was calculated.
Results
CMBs were found in 16 (29%) of patients with IIH for both readers with excellent interobserver agreement (K = 0.8, p = 0.001). There was excellent interobserver agreement for location (K = 0.8, p = 0.001) and side of CMBs (K = 0.8, p = 0.001). There was good interobserver agreement for size of CMBs (K = 0.75, p = 0.002) and number (K = 0.6, p = 0.006). The mean GRS for headache in patients with CMBs (5.61 ± 1.3) was significantly higher (p = 0.02) than that of patients without CMBs (4.9 ± 0.8).
Conclusion
We concluded that SWI can detect CMBs in patients with IIH especially in patients with higher GRS for headache.



Similar content being viewed by others
Abbreviations
- CMBs:
-
Cerebral microbleeds
- IIH:
-
Idiopathic intracranial hypertension
- GRS:
-
Graphic rating scale
References
Radojicic A, Vukovic-Cvetkovic V, Pekmezovic T, Trajkovic G, Zidverc-Trajkovic J, Jensen RH. Predictive role of presenting symptoms and clinical findings in idiopathic intracranial hypertension. J Neurol Sci. 2019;399:89–93.
Wall M. Update on idiopathic intracranial hypertension. Neurol Clin. 2017;35:45–57.
Chan JW. Current concepts and strategies in the diagnosis and management of idiopathic intracranial hypertension in adults. J Neurol. 2017;264:1622–33.
Markey KA, Mollan SP, Jensen RH, Sinclair AJ. Understanding idiopathic intracranial hypertension: mechanisms, management, and future directions. Lancet Neurol. 2016;15:78–91.
Friedman DI. Headaches in idiopathic intracranial hypertension. J Neuroophthalmol. 2019;39:82–93.
Mollan SP, Hoffmann J, Sinclair AJ. Advances in the understanding of headache in idiopathic intracranial hypertension. Curr Opin Neurol. 2019;32:92–8.
Sina F, Razmeh S, Habibzadeh N, Zavari A, Nabovvati M. Migraine headache in patients with idiopathic intracranial hypertension. Neurol Int. 2017;9:7280.
Bidot S, Saindane AM, Peragallo JH, Bruce BB, Newman NJ, Biousse V. Brain imaging in idiopathic intracranial hypertension. J Neuroophthalmol. 2015;35:400–11.
Zur D, Anconina R, Kesler A, Lublinsky S, Toledano R, Shelef I. Quantitative imaging biomarkers for dural sinus patterns in idiopathic intracranial hypertension. Brain Behav. 2017;7:e00613.
Degnan AJ, Levy LM. Pseudotumor cerebri: brief review of clinical syndrome and imaging findings. AJNR Am J Neuroradiol. 2011;32:1986–93.
Razek AAKA, Batouty N, Fathy W, Bassiouny R. Diffusion tensor imaging of the optic disc in idiopathic intracranial hypertension. Neuroradiology. 2018;60:1159–66.
Halefoglu AM, Yousem DM. Susceptibility weighted imaging: clinical applications and future directions. World J Radiol. 2018;10:30–45.
Kurz FT, Freitag M, Schlemmer HP, Bendszus M, Ziener CH. Principles and applications of susceptibility weighted imaging. Radiologe. 2016;56:124–36.
Di Ieva A, Lam T, Alcaide-Leon P, Bharatha A, Montanera W, Cusimano MD. Magnetic resonance susceptibility weighted imaging in neurosurgery: current applications and future perspectives. J Neurosurg. 2015;123:1463–75.
Haller S, Vernooij MW, Kuijer JPA, Larsson EM, Jäger HR, Barkhof F. Cerebral microbleeds: imaging and clinical significance. Radiology. 2018;287:11–28.
Humphries TJ, Mathew P. Cerebral microbleeds: hearing through the silence—a narrative review. Curr Med Res Opin. 2018;35:1–15.
Yeoh H, Lee JY, Lee YJ, Park DW, Kim TY, Ahn GY, et al. Relationship between cerebral microbleeds and white matter MR hyperintensities in systemic lupus erythematosus: a retrospective observational study. Neuroradiology. 2019;61:265–74.
Gregoire SM, Chaudhary UJ, Brown MM, Yousry TA, Kallis C, Jäger HR, et al. The Microbleed Anatomical Rating Scale (MARS): reliability of a tool to map brain microbleeds. Neurology. 2009;73:1759–66.
Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81:1159–65.
Haefeli M, Achim E. Pain assessment. Eur Spine J. 2006;15(1):17–24.
Lenck S, Radovanovic I, Nicholson P, Hodaie M, Krings T, Mendes-Pereira V. Idiopathic intracranial hypertension: the veno glymphatic connections. Neurology. 2018;91:515–22.
Bezerra MLS, Ferreira ACAF, de Oliveira-Souza R. Pseudotumor Cerebri and glymphatic dysfunction. Front Neurol. 2018;8:734.
Madriz Peralta G, Cestari DM. An update of idiopathic intracranial hypertension. Curr Opin Ophthalmol. 2018;29:495–502.
Tsushima Y, Tanizaki Y, Aoki J, Endo K. MR detection of microhemorrhages in neurologically healthy adults. Neuroradiology. 2002;44:31–6.
Abdel Razek AA, Alvarez H, Bagg S, Refaat S, Castillo M. Imaging spectrum of CNS vasculitis. Radiographics. 2014;34:873–94.
Huang X, Lu T, Guo Z, Wei L, Chen S, Qiu W, et al. Susceptibility-weighted imaging in the differential diagnosis of autoimmune central nervous system vasculitis and multiple sclerosis. Mult Scler Relat Disord. 2019;33:70–4.
McKinney AM, Sarikaya B, Gustafson C, Truwit C. Detection of microhemorrhage in posterior reversible encephalopathy syndrome using susceptibility-weighted imaging. AJNR Am J Neuroradiol. 2012;33:896–903.
Suzuki T, Okamoto K, Genkai N, Kakita A, Abe H. A homogeneously enhancing mass evolving into multiple hemorrhagic and necrotic lesions in amoebic encephalitis with necrotizing vasculitis. Clin Imaging. 2019;60:48–52.
Abdel Razek AAK. Routine and advanced diffusion imaging modules of the salivary glands. Neuroimaging Clin N Am. 2018;28:245–54.
Sepahdari AR, Politi LS, Aakalu VK, Kim HJ, Razek AA. Diffusion-weighted imaging of orbital masses: multi-institutional data support a 2-ADC threshold model to categorize lesions as benign, malignant, or indeterminate. AJNR Am J Neuroradiol. 2014;35:170–5.
Razek AAKA, El-Serougy L, Abdelsalam M, Gaballa G, Talaat M. Differentiation of residual/recurrent gliomas from postradiation necrosis with arterial spin labeling and diffusion tensor magnetic resonance imaging-derived metrics. Neuroradiology. 2018;60:169–77.
El-Serougy L, Abdel Razek AA, Ezzat A, Eldawoody H, El-Morsy A. Assessment of diffusion tensor imaging metrics in differentiating low-grade from high-grade gliomas. Neuroradiol J. 2016;29:400–7.
Khalek Abdel Razek AA, El-Serougy L, Abdelsalam M, Gaballa G, Talaat M. Differentiation of primary central nervous system lymphoma from glioblastoma: quantitative analysis using arterial-spin labeling and diffusion tensor imaging. World Neurosurg. 2019;123:303–9.
Razek AA, Nada N. Correlation of choline/creatine and apparent diffusion coefficient values with the prognostic parameters of head and neck squamous cell carcinoma. NMR Biomed. 2016;29:483–9.
Funding
No funding was provided for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Razek, A.A.K.A., Batouty, N.M. & Azab, A.G. Role of susceptibility-weighted imaging in patients with idiopathic intracranial hypertension. Jpn J Radiol 38, 740–745 (2020). https://doi.org/10.1007/s11604-020-00959-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-020-00959-9