Abstract
Purpose
To evaluate the usefulness of high-temporal-resolution dynamic contrast-enhanced (DCE) MRI and quantitative pharmacokinetic analysis to differentiate the normal-appearing pituitary gland from a pituitary macroadenoma.
Materials and methods
Twenty-seven patients with macroadenomas underwent preoperative DCE-MRI with a temporal resolution of 5 s using compressed sensing to obtain pharmacokinetic parameters. Two independent observers localized the normal-appearing pituitary gland on post-contrast T1-weighted images before and after referring to the corresponding Ktrans maps. Agreements between the localizations and intraoperative findings were evaluated using the kappa statistics. The Mann–Whitney U test was used to compare the pharmacokinetic parameters of the normal-appearing pituitary gland and adenoma.
Results
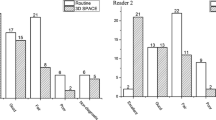
For both observers, the agreement between the MRI-based localization and the intraoperative findings increased after referring to the Ktrans maps (observer 1, 0.930–1; observer 2, 0.636–0.855). The normal-appearing pituitary gland had significantly higher Ktrans [/min] (1.50 ± 0.80 vs 0.58 ± 0.49, P < 0.0001), kep [/min] (3.19 ± 1.29 vs 2.15 ± 1.18, P = 0.0049), and ve (0.43 ± 0.15 vs 0.25 ± 0.17, P = 0.0003) than adenoma.
Conclusion
High-temporal-resolution DCE-MRI and quantitative pharmacokinetic analysis help accurately localize the normal-appearing pituitary gland in patients with macroadenomas. The normal-appearing pituitary gland was characterized by higher Ktrans, kep, and ve than macroadenoma.
Condensed abstract
Dynamic contrast-enhanced MRI with high-temporal-resolution using compressed sensing was used for quantitative pharmacokinetic analysis of pituitary macroadenomas. An observer study, the use of Ktrans maps improved accuracy in localizing the normal-appearing pituitary gland. As compared to an adenoma, the normal-appearing pituitary gland had significantly higher Ktrans, kep, and ve values.



Similar content being viewed by others
References
Ciric I, Ragin A, Baumgartner C, Pierce D. Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience. Neurosurgery. 1997;40:225–37.
Agam MS, Zada G. Complications associated With transsphenoidal pituitary surgery: review of the literature. Neurosurgery. 2018;65:69–73. https://doi.org/10.1093/neuros/nyy160.
Fatemi N, Dusick JR, Mattozo C, McArthur DL, Cohan P, Boscardin J, et al. Pituitary hormonal loss and recovery after transsphenoidal adenoma removal. Neurosurgery. 2008;63:709–19. https://doi.org/10.1227/01.NEU.0000325725.77132.90.
Miki Y, Matsuo M, Nishizawa S, Kuroda Y, Keyaki A, Makita Y, et al. Pituitary adenomas and normal pituitary tissue: enhancement patterns on gadopentetate-enhanced MR imaging. Radiology. 1990;177:35–8.
Lee HB, Kim ST, Kim HJ, Kim KH, Jeon P, Byun HS, et al. Usefulness of the dynamic gadolinium-enhanced magnetic resonance imaging with simultaneous acquisition of coronal and sagittal planes for detection of pituitary microadenomas. Eur Radiol. 2012;22:514–8. https://doi.org/10.1007/s00330-011-2291-3.
Petrillo A, Fusco R, Petrillo M, Granata V, Bianco F, Di Marzo M, et al. DCE-MRI time-intensity curve visual inspection to assess pathological response after neoadjuvant therapy in locally advanced rectal cancer. Jpn J Radiol. 2018;36:611–21. https://doi.org/10.1007/s11604-018-0760-1.
Lloyd RV, Scheithauer BW, Kuroki T, Vidal S, Kovacs K, Stefaneanu L. Vascular endothelial growth factor (VEGF) expression in human pituitary adenomas and carcinomas. Endocr Pathol. 1999;10:229–35.
Jasek E, Furgal-Borzych A, Lis GJ, Litwin JA, Rzepecka-Wozniak E, Trela F. Microvessel density and area in pituitary microadenomas. Endocr Pathol. 2009;20:221–6. https://doi.org/10.1007/s12022-009-9091-1.
Sourbron SP, Buckley DL. On the scope and interpretation of the Tofts models for DCE-MRI. Magn Reson Med. 2011;66:735–45. https://doi.org/10.1002/mrm.22861.
Zhang N, Zhang L, Qiu B, Meng L, Wang X, Hou BL. Correlation of volume transfer coefficient Ktrans with histopathologic grades of gliomas. J Magn Reson Imaging. 2012;36:355–63. https://doi.org/10.1002/jmri.23675.
Lu S, Gao Q, Yu J, Li Y, Cao P, Shi H, et al. Utility of dynamic contrast-enhanced magnetic resonance imaging for differentiating glioblastoma, primary central nervous system lymphoma and brain metastatic tumor. Eur J Radiol. 2016;85:1722–7. https://doi.org/10.1016/j.ejrad.2016.07.005.
Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223–32.
Jia ZZ, Gu HM, Zhou XJ, Shi JL, Li MD, Zhou GF, et al. The assessment of immature microvascular density in brain gliomas with dynamic contrast-enhanced magnetic resonance imaging. Eur J Radiol. 2015;84:1805–9. https://doi.org/10.1016/j.ejrad.2015.05.035.
Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007;58:1182–95.
Rofsky NM, Lee VS, Laub G, Pollack MA, Krinsky GA, Thomasson D, et al. Abdominal MR imaging with a volumetric interpolated breath-hold examination. Radiology. 1999;212:876–84.
Osamura RY, Lopes MBS, Grossman A, Kontogeorgos G, Trouillas J. Tumor of the pituitary gland. In: Lloyd RV, Osamura RY, Kloppel G, Rosai J, editors. WHO Classification of Tumours of Endocrine Organs. 4th ed. Lyon: IARC; 2017. p. 11–64.
Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–8.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45.
Rossi Espagnet MC, Bangiyev L, Haber M, Block KT, Babb J, Ruggiero V, et al. High-resolution DCE-MRI of the pituitary gland using radial k-space acquisition with compressed sensing reconstruction. AJNR Am J Neuroradiol. 2015;36:1444–9. https://doi.org/10.3174/ajnr.A4324.
Zhai J, Zheng W, Zhang Q, Wu J, Zhang X. Pharmacokinetic analysis for the differentiation of pituitary microadenoma subtypes through dynamic contrast-enhanced magnetic resonance imaging. Oncol Lett. 2019;17:4237–44. https://doi.org/10.3892/ol.2019.10083.
Kamimura K, Nakajo M, Yoneyama T, Fukukura Y, Fujio S, Goto Y, et al. Assessment of microvessel perfusion of pituitary adenomas: a feasibility study using turbo spin-echo-based intravoxel incoherent motion imaging. AJNR Am J Neuroradiol. 2019. https://doi.org/10.1007/s00330-019-06443-x.
Zhao M, Guo LL, Huang N, Wu Q, Zhou L, Zhao H, et al. Quantitative analysis of permeability for glioma grading using dynamic contrast-enhanced magnetic resonance imaging. Oncol Lett. 2017;14:5418–26. https://doi.org/10.3892/ol.2017.6895.
Zou HH, Yu J, Wei Y, Wu JF, Xu Q. Response to neoadjuvant chemoradiotherapy for locally advanced rectum cancer: Texture analysis of dynamic contrast-enhanced MRI. J Magn Reson Imaging. 2019;49:885–93. https://doi.org/10.1002/jmri.26254.
Sahoo P, Frankel P, Ressler J, Gutova M, Annala AJ, Badie B, et al. Early changes in tumor perfusion from T1-weighted dynamic contrast-enhanced MRI following neural stem cell-mediated therapy of recurrent high-grade glioma correlate with overall survival. Stem Cells Int. 2018. https://doi.org/10.1155/2018/5312426.
Sen R, Sen C, Pack J, Block KT, Golfinos JG, Prabhu V, et al. Role of high-resolution dynamic contrast-enhanced MRI with golden-angle radial sparse parallel reconstruction to identify the normal pituitary gland in patients with macroadenomas. AJNR Am J Neuroradiol. 2017;38:1117–21. https://doi.org/10.3174/ajnr.A5244.
Acknowledgements
This paper was presented at the 78th annual meeting of the Japan Radiological Society (JRS) in Yokohama, Japan, in 2019. The authors wish to thank the staff of Kagoshima University Hospital for their support. The authors would like to thank Enago (www.enago.jp) for the English language review.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical statement
The study protocol was approved by the Ethics Committee of Kagoshima University Graduate School of Medical and Dental Sciences (approval no. 180255) and was conducted in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kamimura, K., Nakajo, M., Yoneyama, T. et al. Quantitative pharmacokinetic analysis of high-temporal-resolution dynamic contrast-enhanced MRI to differentiate the normal-appearing pituitary gland from pituitary macroadenoma . Jpn J Radiol 38, 649–657 (2020). https://doi.org/10.1007/s11604-020-00942-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-020-00942-4