Abstract
Background
The purpose of this study is to investigate the performance of dual-layer detector spectral CT for iron deposition compared to magnetic resonance imaging (MRI) T2* imaging.
Methods
Thirty-one patients with a clinical history of myelodysplastic syndromes and aplastic anemia underwent liver and cardiac T2*-weighted unenhanced MRI on a three-tesla MRI scanner, and underwent unenhanced CT scan laterally on a 128-row spectral detector CT. R2* values of the liver, septal muscle, and paraspinal muscle were calculated. Attenuation differences (ΔH) in the liver and myocardium were calculated between the lower (50 keV) and higher (120 keV) energy levels.
Results
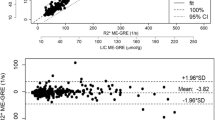
The liver and cardiac T2* values were 9.54 ± 5.63 ms and 21.41 ± 2.44 ms, respectively. The liver-to-muscle and myocardium-to-muscle T2* value ratios were 0.37 ± 0.23 and 0.79 ± 0.19, respectively. The liver and cardiac ΔH were − 1.13 ± 4.24 HU and 2.22 ± 4.41 HU, respectively. There was a strong linear correlation between the liver R2* and ΔH (r = − 0.832, P < 0.001), but weak correlation existed between the cardiac R2* and ΔH (P = 0.041).
Conclusions
Dual-layer detector spectral unenhanced CT seemed to be equally valuable to MRI T2* imaging for evaluating liver iron overload.



Similar content being viewed by others
References
Porter J, de Witte T, Cappellini M, Gattermann N. New insights into transfusion-related iron toxicity: implications for the oncologist. Crit Rev Oncol Hematol. 2016;99(10):261–71.
Gautam R, Wallace DF, Nathan SV. Hepcidin: regulation of the master iron regulator. Biosci Rep. 2015;35(3):e00192.
Jabbour E, Ghanem H, Huang X, Ravandi F, Garciamanero G, O'Brien S, et al. Acute myeloid leukemia after myelodysplastic syndrome and failure of therapy with hypomethylating agents: an emerging entity with a poor prognosis. Clin Lymphoma Myeloma Leuk. 2014;14(2):93–7.
Labranche R, Gilbert G, Cerny M, Vu K-N, Soulières D, Olivié D, et al. Liver iron quantification with MR imaging: a primer for radiologists. RadioGraphics. 2018;38(2):392–412.
Coates TD. Physiology and pathophysiology of iron in hemoglobin-associated diseases. Free Radic Biol Med. 2014;72(6):23–40.
Pennell DJ, Udelson JE, Arai AE, Bozkurt B, Cohen AR, Galanello R, et al. Cardiovascular function and treatment in β-thalassemia major: a consensus statement from the American Heart Association. Circulation. 2013;128(13):E203.
Krittayaphong R, Zhang S, Saiviroonporn P, Viprakasit V, Tanapibunpon P, Komoltri C, et al. Detection of cardiac iron overload with native magnetic resonance T1 and T2 mapping in patients with thalassemia. Int J Cardiol. 2017;248(5):421–6.
He T. Cardiovascular magnetic resonance T2* for tissue iron assessment in the heart. Quant Imaging Med Surg. 2014;4(5):407–12.
Chirico V, Rigoli L, Lacquaniti A, Salpietro V, Piraino B, Amorini M, et al. Endocrinopathies, metabolic disorders, and iron overload in major and intermedia thalassemia: serum ferritin as diagnostic and predictive marker associated with liver and cardiac T2* MRI assessment. Eur J Haematol. 2015;94(5):404–12.
Ibrahim E-SH, Bowman AW. Characterization of myocardial iron overload by dual-energy computed tomography compared to T2* MR. A phantom study. In: Conf Proc IEEE Eng Med Biol Soc pp. 5133–6. 2014.
Luo XF, Yang Y, Yan J, Xie XQ, Zhang H, Chai WM, et al. Virtual iron concentration imaging based on dual-energy CT for noninvasive quantification and grading of liver iron content: an iron overload rabbit model study. Eur Radiol. 2015;25(9):2657–64.
Tsai YS, Chen JS, Wang CK, Lu CH, Cheng CN, Kuo CS, et al. Quantitative assessment of iron in heart and liver phantoms using dual-energy computed tomography. Exp Ther Med. 2014;8(3):907–12.
Ibrahim E-SH, Bowman AW, Khalifa AM. Dual-energy computed tomography versus magnetic resonance imaging for the assessment of iron overload. In: 2nd Middle East Conference on Biomedical Engineering. 2014: 5–8.
Fischer MA, Reiner CS, Raptis D, Donati O, Goetti R, Clavien PA, et al. Quantification of liver iron content with CT-added value of dual-energy. Eur Radiol. 2011;21(8):1727–32.
Majd Z, Haghpanah S, Ajami GH, Matin S, Namazi H, Bardestani M, et al. Serum ferritin levels correlation with Heart and liver MRI and LIC in patients with transfusion-dependent thalassemia. Iran Red Crescent Med J. 2015;17(4):e24959.
Azarkeivan A, Hashemieh M, Akhlaghpoor S, Shirkavand A, Yaseri M, Sheibani K. Relation between serum ferritin and liver and heart MRI T2* in beta thalassaemia major patients. East Mediterr Health J. 2013;19(8):727–32.
Felipe A, Guadalupe E, Druso P, Carlos M, Pablo S, Oscar C, et al. Serum ferritin is associated with metabolic syndrome and red meat consumption. Oxidative Med Cell Longev. 2015;5:769–39.
Sharma S, Fischer R, Schoennagel B, Nielsen P, Kooijman H, Yamamura J, et al. MRI-based quantitative susceptibility mapping (QSM) and R2* mapping of liver iron overload: comparison with SQUID-based biomagnetic liver susceptometry. Magn Reson Med. 2017;78(1):264–70.
Chan WC, Tejani Z, Budhani F, Massey C, Haider MA. R2* as a surrogate measure of FerriScan iron quantification in thalassemia. J Magn Reson Imaging. 2014;39(4):1007–111.
Nienhuis AW, Nathan DG. Pathophysiology and clinical manifestations of the β-thalassemias. Cold Spring Harbor Perspect Med. 2012;2(12):a011726.
Hernando D, Levin YS, Sirlin CB, Reeder SB. Quantification of liver iron with MRI: state of the art and remaining challenges. J Magn Reson Imaging. 2014;40(5):1003–211.
Storey P, Thompson AA, Carqueville CL, de Freitas RA, Rigsby CK. R2* imaging of transfusional iron burden at 3 T and comparison with 1.5 T. J Magn Reson Imaging. 2007;25(3):540–7.
Wood JC, Enriquez C, Ghugre N, Tyzka JM, Carson S, Nelson MD, et al. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106(4):1460–5.
Hernando D, Kramer JH, Reeder SB. Multipeak fat-corrected complex R2* relaxometry: theory, optimization, and clinical validation. Magn Reson Med. 2013;70(5):1319–31.
Abadia AF, Grant KL, Carey KE, Bolch WE, Morin RL. Spatial distribution of iron within the normal human liver using dual-source dual-energy CT imaging. Investig Radiol. 2017;52(11):693–700.
Luo XF, Xie XQ, Cheng S, Yang Y, Yan J, Zhang H, et al. Dual-energy CT for patients suspected of having liver iron overload: can virtual iron content imaging accurately quantify liver iron content? Radiology. 2015;277(1):95–103.
Joe E, Kim SH, Lee KB, Jang JJ, Lee JY, Lee JM. Feasibility and accuracy of dual-source dual-energy CT for noninvasive determination of hepatic iron accumulation. Int J Med Radiol. 2012;262(1):126–35.
Ma J, Song ZQ, Yan FH. Separation of hepatic iron and fat by dual-source dual-energy computed tomography based on material decomposition: an animal study. PLoS ONE. 2014;9(10):e110964.
Funding
The study was funded by 345 Talent Project in Shengjing Hospital of China Medical University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
I certify that this manuscript is original and has not been published and will not be submitted elsewhere for publication while being considered by Japanese Journal of Radiology.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ma, Q., Hu, J., Yang, W. et al. Dual-layer detector spectral CT versus magnetic resonance imaging for the assessment of iron overload in myelodysplastic syndromes and aplastic anemia. Jpn J Radiol 38, 374–381 (2020). https://doi.org/10.1007/s11604-020-00921-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-020-00921-9