Abstract
Purpose
To explore the regional spontaneous neural activity and functional connectivity alterations of adjustment disorder (AD) in new recruits with in vivo resting-state functional MR (rs-fMRI).
Materials and methods
Resting-state fMRI was performed in 31 recruits with AD and in 31 control recruits. Regional homogeneity (ReHo) was used to detect the regional synchronizing features of neuronal activations. Correlative analysis was performed to investigate the relationship between the Symptom Check List-90 (SCL-90) score and ReHo in regions with significant group differences. Regions with significant correlation were then defined as regions of interest (ROIs), and seed-ROI based whole-brain functional connectivity was performed.
Results
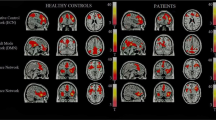
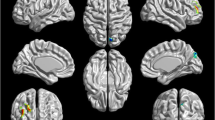
Compared with the controls, patients with AD had significantly lower ReHo in the left posterior cerebellar lobe, bilateral medial orbitofrontal cortex, bilateral caudate and left middle temporal gyrus, whereas regions with enhanced ReHo were confined to bilateral posterior cingulate gyrus/precuneus. Only the left posterior cerebellar lobe showed significant correlation between ReHo and the SCL-90 score, and was defined as the seed ROI. Decreased functional connectivity was found between the ROI and bilateral supplementary motor area.
Conclusion
This study reveals abnormalities in recruits with AD in baseline brain function activities, which could further improve our understanding of the neural substrates of cognitive impairment in AD.




Similar content being viewed by others
References
Mittal VA, Walker EF. Diagnostic and statistical manual of mental disorders. Psychiatry Res. 2011;189(1):158–9.
Pelkonen M, Marttunen M, Henriksson M, Lonnqvist J. Suicidality in adjustment disorder—clinical characteristics of adolescent outpatients. Eur Child Adolesc Psychiatry. 2005;14(3):174–80.
Hund B, Reuter K, Harter M, Brahler E, Faller H, Keller M, et al. Stressors, symptom profile, and predictors of adjustment disorder in cancer patients. Results from an epidemiological study with the composite international diagnostic interview, adaptation for oncology (Cidi-O). Depress Anxiety. 2016;33(2):153–61.
Kovacs M, Gatsonis C, Pollock M, Parrone PL. A controlled prospective study of DSM-III adjustment disorder in childhood. Short-term prognosis and long-term predictive validity. Arch Gen Psychiatry. 1994;51(7):535–41.
De Hepcee C, Reynaert C, Jacques D, Zdanowicz N. Suicide in adolescence: attempt to cure a crisis, but also the fatal outcome of certain pathologies. Psychiatr Danub. 2015;27(Suppl 1):296–9.
Monahan P, Hu Z, Rohrbeck P. Mental disorders and mental health problems among recruit trainees, US Armed Forces, 2000–2012. MSMR. 2013;20(7):13–8 (discussion 6–8).
JunSheng Z, Kai W, JiaTong W, Wei X. Investigation on mental health and survival ability of recruits. J Fourth Mil Med Univ. 2009;30(11):1045–7.
Lung FWLF, Shu BC. The premorbid personality in military students with adjustment disorder. Mil Psychol. 2006;18:77–88.
Derogatis LR, Cleary PA. Confirmation of the dimensional structure of the SCL-90: a study in construct validation. J Clin Psychol. 1977;33(4):981–9.
Zhang J, Zhang X. Chinese college students’ SCL-90 scores and their relations to the college performance. Asian J Psychiatry. 2013;6(2):134–40.
Kumano H, Ida I, Oshima A, Takahashi K, Yuuki N, Amanuma M, et al. Brain metabolic changes associated with predisposition to onset of major depressive disorder and adjustment disorder in cancer patients—a preliminary PET study. J Psychiatr Res. 2007;41(7):591–9.
Jeong HG, Ko YH, Han C, Kim YK, Joe SH. Distinguishing quantitative electroencephalogram findings between adjustment disorder and major depressive disorder. Psychiatry investigation. 2013;10(1):62–8.
Di X, Biswal BB, Alzheimer’s Disease Neuroimaging I. Metabolic brain covariant networks as revealed by FDG-PET with reference to resting-state fMRI networks. Brain Connect. 2012;2(5):275–83.
Yao Z, Wang L, Lu Q, Liu H, Teng G. Regional homogeneity in depression and its relationship with separate depressive symptom clusters: a resting-state fMRI study. J Affect Disord. 2009;115(3):430–8.
Ke M, Zou R, Shen H, Huang X, Zhou Z, Liu Z. Bilateral functional asymmetry disparity in positive and negative schizophrenia revealed by resting-state fMRI. Psychiatry Res Neuroimaging. 2010;182(1):30–9.
Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22(1):394–400.
Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29(2):83–91.
Albert NB, Robertson EM, Miall RC. The resting human brain and motor learning. Curr Biol. 2009;19(12):1023–7.
Zuo X-N, Xu T, Jiang L, Yang Z, Cao X-Y, He Y, et al. Toward reliable characterization of functional homogeneity in the human brain: preprocessing, scan duration, imaging resolution and computational space. NeuroImage. 2013;65:374–86.
Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102(27):9673–8.
Ledberg A, Akerman S, Roland PE. Estimation of the probabilities of 3D clusters in functional brain images. Neuroimage. 1998;8(2):113–28.
Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216.
Chen J, Fan C, Li J, Han Q, Lin J, Yang T, et al. Increased intraregional synchronized neural activity in adult brain after prolonged adaptation to high-altitude hypoxia: a resting-state fMRI study. High Alt Med Biol. 2016;17(1):16–24.
Paakki JJ, Rahko J, Long X, Moilanen I, Tervonen O, Nikkinen J, et al. Alterations in regional homogeneity of resting-state brain activity in autism spectrum disorders. Brain Res. 2010;1321:169–79.
Ke M, Zou R, Shen H, Huang X, Zhou Z, Liu Z, et al. Bilateral functional asymmetry disparity in positive and negative schizophrenia revealed by resting-state fMRI. Psychiatry Res. 2010;182(1):30–9.
Zhang Z, Liu Y, Jiang T, Zhou B, An N, Dai H, et al. Altered spontaneous activity in Alzheimer’s disease and mild cognitive impairment revealed by regional homogeneity. Neuroimage. 2012;59(2):1429–40.
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98(2):676–82.
Grimm S, Boesiger P, Beck J, Schuepbach D, Bermpohl F, Walter M, et al. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology. 2009;34(4):932–43.
Marchand WR, Lee JN, Johnson S, Thatcher J, Gale P, Wood N, et al. Striatal and cortical midline circuits in major depression: implications for suicide and symptom expression. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36(2):290–9.
Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012;71(4):286–93.
Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annu Rev Neurosci. 2002;25:563–93.
Koziol LF, Budding D, Andreasen N, D’Arrigo S, Bulgheroni S, Imamizu H, et al. Consensus paper: the cerebellum’s role in movement and cognition. Cerebellum. 2014;13(1):151–77.
Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7(7):511–22.
Leiner HC, Leiner AL, Dow RS. Cognitive and language functions of the human cerebellum. Trends Neurosci. 1993;16(11):444–7.
Hart H, Rubia K. Neuroimaging of child abuse: a critical review. Front Hum Neurosci. 2012;6:52.
Lee HY, Tae WS, Yoon HK, Lee BT, Paik JW, Son KR, et al. Demonstration of decreased gray matter concentration in the midbrain encompassing the dorsal raphe nucleus and the limbic subcortical regions in major depressive disorder: an optimized voxel-based morphometry study. J Affect Disord. 2011;133(1–2):128–36.
Yin Y, Li L, Jin C, Hu X, Duan L, Eyler LT, et al. Abnormal baseline brain activity in posttraumatic stress disorder: a resting-state functional magnetic resonance imaging study. Neurosci Lett. 2011;498(3):185–9.
Wei XH, Ren JL, Liu WH, Yang RM, Xu XD, Liu J, et al. Increased interhemispheric functional connectivity in college students with non-clinical depressive symptoms in resting state. Neurosci Lett. 2015;589:67–72.
Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48(8):813–29.
Maddock RJ. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci. 1999;22(7):310–6.
Zhang YQM, Xie B, et al. Brain’s default network in patients with post-traumatic stress disorder at resting state. J Third Mil Med Univ. 2011;33:2271–3.
Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, et al. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci USA. 2009;106(47):20069–74.
Yin Y, Jin C, Eyler LT, Jin H, Hu X, Duan L, et al. Altered regional homogeneity in post-traumatic stress disorder: a resting-state functional magnetic resonance imaging study. Neurosci Bull. 2012;28(5):541–9.
Brunet E, Sarfati Y, Hardy-Bayle MC, Decety J. A PET investigation of the attribution of intentions with a nonverbal task. Neuroimage. 2000;11(2):157–66.
Zhong WJ, Zhou ZM, Zhao JN, Wu W, Guo DJ. Abnormal spontaneous brain activity in minimal hepatic encephalopathy: resting-state fMRI study. Diagn Interv Radiol. 2016;22(2):196–200.
Hund B, Reuter K, Harter M, Brahler E, Faller H, Keller M, et al. Stressors, symptom profile, and predictors of adjustment disorder in cancer patients. Results from an epidemiological study with the composite international diagnostic interview, adaptation for oncology (Cidi-O). Depress Anxiety. 2016;33(2):153–61.
Acknowledgements
This research was supported by Grand of Nanjing Command, PLA, China (10Z030).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This study was approved by the ethics committee and the institutional review board of Fuzhou General Hospital, Second Military Medical University. All patients provided written informed consent.
Additional information
Co-first author: Y. Lin.
About this article
Cite this article
Li, H., Lin, Y., Chen, J. et al. Abnormal regional homogeneity and functional connectivity in adjustment disorder of new recruits: a resting-state fMRI study. Jpn J Radiol 35, 151–160 (2017). https://doi.org/10.1007/s11604-017-0614-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-017-0614-2