Abstract
Objective
Rifaximin is an effective component of treatment strategies for liver and intestinal diseases. However, the efficacy of rifaximin in hepatic sinusoidal obstruction syndrome (HSOS) has not been explored. The present study aimed to investigate the efficacy and mechanism of rifaximin in HSOS.
Methods
An HSOS model was established in mice through the administration of monocrotaline (MCT, 800 mg/kg), and part of the HSOS mice were intragastrically administered with rifaximin. Then, the efficacy of rifaximin in HSOS was evaluated based on the liver pathological findings, liver proinflammatory cytokines, and alanine aminotransferase and aspartate aminotransferase levels. The Ussing chamber was used to evaluate the intestinal permeability, and tight junction (TJ) proteins were measured by Western blotting and real-time polymerase chain reaction to evaluate the intestinal barrier integrity. Then, the serum proinflammatory cytokine levels were evaluated by enzyme-linked immunosorbent assay. Afterwards, an in vitro experiment was performed to determine the relationship between rifaximin and TJ proteins.
Results
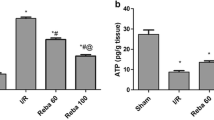
Rifaximin effectively alleviated the MCT-induced HSOS liver injury, suppressed the expression of liver proinflammatory cytokines, and reduced the serum levels of tumor necrosis factor-alpha and interleukin-6. Furthermore, rifaximin reduced the intestinal permeability, improved the intestinal barrier integrity, and promoted the expression of TJ proteins.
Conclusion
The results revealed that the intestinal barrier integrity was destroyed in MCT-induced HSOS. The significant alleviation of MCT-induced HSOS induced by rifaximin might be correlated to the repairment of intestinal barrier integrity via the regulation of the TJ protein expression.
Similar content being viewed by others
References
Mohty M, Malard F, Abecassis M, et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant, 2016,51(7):906–912
Yakushijin K, Atsuta Y, Doki N, et al. Sinusoidal obstruction syndrome after allogeneic hematopoietic stem cell transplantation: Incidence, risk factors and outcomes. Bone Marrow Transplant, 2016,51(3):403–409
Dignan FL, Wynn RF, Hadzic N, et al. BCSH/BSBMT guideline: diagnosis and management of veno-occlusive disease (sinusoidal obstruction syndrome) following haematopoietic stem cell transplantation. Br J Haematol, 2013,163(4):444–457
Dai N, Yu YC, Ren TH, et al. Gynura root induces hepatic veno-occlusive disease: a case report and review of the literature. World J Gastroenterol, 2007,13(10):1628–1631
EASL Clinical Practice Guidelines: Drug-induced liver injury. J Hepatol, 2019,70(6):1222–1261
Yang XQ, Ye J, Li X, et al. Pyrrolizidine alkaloids-induced hepatic sinusoidal obstruction syndrome: Pathogenesis, clinical manifestations, diagnosis, treatment, and outcomes. World J Gastroenterol, 2019, 25(28):3753–3763
de Lédinghen V, Villate A, Robin M, et al. Sinusoidal obstruction syndrome. Clin Res Hepatol Gastroenterol, 2020,44(4):480–485
Fan CQ, Crawford JM. Sinusoidal obstruction syndrome (hepatic veno-occlusive disease). J Clin Exp Hepatol, 2014,4(4):332–346
Valla DC, Cazals-Hatem D. Sinusoidal obstruction syndrome. Clin Res Hepatol Gastroenterol, 2016,40(4): 378–385
Harb R, Xie G, Lutzko C, et al. Bone marrow progenitor cells repair rat hepatic sinusoidal endothelial cells after liver injury. Gastroenterology, 2009,137(2):704–712
Xiao L, Hu L, Chu H, et al. Retrorsine Cooperates with Gut Microbiota to Promote Hepatic Sinusoidal Obstruction Syndrome by Disrupting the Gut Barrier. J Clin Transl Hepatol, 2022,10(6):1086–1098
Tripathi A, Debelius J, Brenner DA, et al. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol, 2018,15(7):397–411
Chopyk DM, Grakoui A. Contribution of the Intestinal Microbiome and Gut Barrier to Hepatic Disorders. Gastroenterology, 2020,159(3):849–863
Wang HJ, Gao B, Zakhari S, et al. Inflammation in alcoholic liver disease. Annu Rev Nutr, 2012,32:343–368
Meijers B, Farré R, Dejongh S, et al. Intestinal Barrier Function in Chronic Kidney Disease. Toxins (Basel), 2018,10(7):298
Suzuki T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim Sci J, 2020, 91(1):e13357
Zeisel MB, Dhawan P, Baumert TF. Tight junction proteins in gastrointestinal and liver disease. Gut, 2019, 68(3):547–561
Gangarapu V, Ince AT, Baysal B, et al. Efficacy of rifaximin on circulating endotoxins and cytokines in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol, 2015,27(7):840–845
Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology, 2005,41(6):1313–1321
Farhadi A, Gundlapalli S, Shaikh M, et al. Susceptibility to gut leakiness: a possible mechanism for endotoxaemia in non-alcoholic steatohepatitis. Liver Int, 2008,28(7):1026–1033
Thuy S, Ladurner R, Volynets V, et al. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J Nutr, 2008,138(8):1452–1455
Bergheim I, Weber S, Vos M, et al. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol, 2008,48(6):983–992
Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med, 2010,362(12):1071–1081
Jin Y, Ren X, Li G, et al. Beneficial effects of Rifaximin in post-infectious irritable bowel syndrome mouse model beyond gut microbiota. J Gastroenterol Hepatol, 2018,33(2):443–452
DeLeve LD, McCuskey RS, Wang X, et al. Characterization of a reproducible rat model of hepatic veno-occlusive disease. Hepatology, 1999,29(6):1779–1791
Ali AH, Carey EJ, Lindor KD. Diagnosis and management of primary biliary cirrhosis. Expert Rev Clin Immunol, 2014, 10(12):1667–1678
Kim S, Kim GH. Roles of claudin-2, ZO-1 and occludin in leaky HK-2 cells. PLoS One, 2017,12(12):e0189221
Prozialeck WC, Edwards JR, Lamar PC, et al. Epithelial barrier characteristics and expression of cell adhesion molecules in proximal tubule-derived cell lines commonly used for in vitro toxicity studies. Toxicol In Vitro, 2006,20(6):942–953
Huang Z, Chen M, Wei M, et al. Liver Inflammatory Injury Initiated by DAMPs-TLR4-MyD88/TRIF-NFκB Signaling Pathway Is Involved in Monocrotaline-Induced HSOS. Toxicol Sci, 2019,172(2):385–397
Glück J, Waizenegger J, Braeuning A, et al. Pyrrolizidine Alkaloids Induce Cell Death in Human HepaRG Cells in a Structure-Dependent Manner. Int J Mol Sci, 2020,22(1):202
Chojkier M. Hepatic sinusoidal-obstruction syndrome: toxicity of pyrrolizidine alkaloids. J Hepatol, 2003, 39(3):437–446
Yang M, Ruan J, Fu PP, et al. Cytotoxicity of pyrrolizidine alkaloid in human hepatic parenchymal and sinusoidal endothelial cells: Firm evidence for the reactive metabolites mediated pyrrolizidine alkaloid-induced hepatotoxicity. Chem Biol Interact, 2016,243:119–126
Teschke R, Vongdala N, Quan NV, et al. Metabolic Toxification of 1,2-Unsaturated Pyrrolizidine Alkaloids Causes Human Hepatic Sinusoidal Obstruction Syndrome: The Update. Int J Mol Sci, 2021,22(19):10419
Huang Z, Jing X, Sheng Y, et al. (-)-Epicatechin attenuates hepatic sinusoidal obstruction syndrome by inhibiting liver oxidative and inflammatory injury. Redox Biol, 2019,22:101117
Huang Z, Zhao Q, Chen M, et al. Liquiritigenin and liquiritin alleviated monocrotaline-induced hepatic sinusoidal obstruction syndrome via inhibiting HSP60-induced inflammatory injury. Toxicology, 2019,428: 152307
Allam-Ndoul B, Castonguay-Paradis S, Veilleux A. Gut Microbiota and Intestinal Trans-Epithelial Permeability. Int J Mol Sci, 2020,21(17):6402
Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes, 2007,56(7):1761–1772
Liao L, Schneider KM, Galvez EJC, et al. Intestinal dysbiosis augments liver disease progression via NLRP3 in a murine model of primary sclerosing cholangitis. Gut, 2019,68(8):1477–1492
Ponziani FR, Zocco MA, Cerrito L, et al. Bacterial translocation in patients with liver cirrhosis: physiology, clinical consequences, and practical implications. Expert Rev Gastroenterol Hepatol, 2018,12(7):641–656
Mani V, Weber TE, Baumgard LH, et al. Growth and Development Symposium: Endotoxin, inflammation, and intestinal function in livestock. J Anim Sci, 2012,90(5):1452–1465
Rice JB, Stoll LL, Li WG, et al. Low-level endotoxin induces potent inflammatory activation of human blood vessels: inhibition by statins. Arterioscler Thromb Vasc Biol, 2003,23(9):1576–1582
Xu Q, Guo J, Li X, et al. Necroptosis Underlies Hepatic Damage in a Piglet Model of Lipopolysaccharide-Induced Sepsis. Front Immunol, 2021,12:633830
Calanni F, Renzulli C, Barbanti M, et al. Rifaximin: beyond the traditional antibiotic activity. J Antibiot (Tokyo), 2014,67(9):667–670
DuPont HL, Jiang ZD, Okhuysen PC, et al. A randomized, double-blind, placebo-controlled trial of rifaximin to prevent travelers’ diarrhea. Ann Intern Med, 2005,142(10):805–812
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
On behalf of all authors, the corresponding author states that there are no conflicts of interest.
Additional information
This study was supported by grants from the National Natural Science Foundation of China (No. 81800480 and NO. 81770582), and the Graduates’ Innovation Fund, Huazhong University of Science and Technology (No. 2021yjsCXCY106).
Rights and permissions
About this article
Cite this article
Shu, Yy., Hu, Ll., Yang, L. et al. Rifaximin Prevents Intestinal Barrier Dysfunction and Alleviates Liver Injury in MCT-induced HSOS Mice. CURR MED SCI 43, 1183–1194 (2023). https://doi.org/10.1007/s11596-023-2801-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-023-2801-y