Abstract
Objective
Numerous schizophrenic patients are suffering from obesity primarily attributed to antipsychotic medication and poor dietary habits. This study investigated the progressive deterioration of olanzapine-induced metabolic disorders in the presence of a high-fat diet (HFD) and explored the involvement of endoplasmic reticulum (ER) stress.
Methods
Female Sprague-Dawley rats fed on a standard chow diet or HFD were treated with olanzapine (3 mg/kg/day) and the ER stress inhibitor 4-phenylbutyric acid (4-PBA, 1 and 0.5 g/kg/day) for 8 days. Changes in body weight, food intake, and plasma lipids were assessed. Hepatic fat accumulation was evaluated using oil red O staining. Western blotting and immunofluorescence assays were employed to examine the expression of ER stress markers, NOD-like receptor pyrin domain-containing protein 3 (NLRP3), and proopiomelanocortin (POMC) in the hypothalamus or liver.
Results
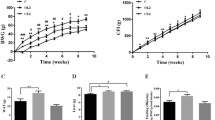
Compared to olanzapine alone, olanzapine+HFD induced greater weight gain, increased hyperlipidemia, and enhanced hepatic fat accumulation (P<0.05). Co-treatment with 4-PBA exhibited a dose-dependent inhibition of these effects (P<0.05). Further mechanistic investigations revealed that olanzapine alone activated ER stress, upregulated NLRP3 expression in the hypothalamus and liver, and downregulated hypothalamic POMC expression. The HFD exacerbated these effects by 50%–100%. Moreover, co-administration of 4-PBA dose-dependently attenuated the olanzapine+HFD-induced alterations in ER stress, NLRP3, and POMC expression in the hypothalamus and liver (P<0.05).
Conclusion
HFD worsened olanzapine-induced weight gain and lipid metabolic disorders, possibly through ER stress-POMC and ER stress-NLRP3 signaling. ER stress inhibitors could be effective in preventing olanzapine+HFD-induced metabolic disorders.
Similar content being viewed by others
References
Olfson M, Gerhard T, Huang C, et al. Premature Mortality Among Adults With Schizophrenia in the United States. JAMA Psychiatry, 2015,72(12):1172–1181
McCreadie RG. Diet, smoking and cardiovascular risk in people with schizophrenia: descriptive study. Br J Psychiatry, 2003,183:534–539
Włodarczyk A, Wiglusz MS, Cubala WJ. Ketogenic diet for schizophrenia: Nutritional approach to antipsychotic treatment. Med Hypotheses, 2018,118:74–77
Dipasquale S, Pariante CM, Dazzan P, et al. The dietary pattern of patients with schizophrenia: a systematic review. J Psychiatr Res, 2013,47(2):197–207
Firth J, Stubbs B, Teasdale SB, et al. Diet as a hot topic in psychiatry: a population-scale study of nutritional intake and inflammatory potential in severe mental illness. World Psychiatry, 2018,17(3):365–367
van Zonneveld SM, Haarman BCM, van den Oever EJ, et al. Unhealthy diet in schizophrenia spectrum disorders. Curr Opin Psychiatry, 2022,35(3):177–185
Chang GR, Liu HY, Yang WC, et al. Clozapine Worsens Glucose Intolerance, Nonalcoholic Fatty Liver Disease, Kidney Damage, and Retinal Injury and Increases Renal Reactive Oxygen Species Production and Chromium Loss in Obese Mice. Int J Mol Sci, 2021,22(13):6680
Fell MJ, Neill JC, Anjum N, et al. Investigation into the influence of a high fat diet on antipsychotic-induced weight gain in female rats. J Psychopharmacol, 2008,22(2):182–186
Isaacson RH, Beier JI, Khoo NK, et al. Olanzapine-induced liver injury in mice: aggravation by high-fat diet and protection with sulforaphane. J Nutr Biochem, 2020,81:108399
Smith GC, Vickers MH, Shepherd PR. Olanzapine effects on body composition, food preference, glucose metabolism and insulin sensitivity in the rat. Arch Physiol Biochem, 2011,117(4):241–249
Townsend LK, Peppler WT, Bush ND, et al. Obesity exacerbates the acute metabolic side effects of olanzapine. Psychoneuroendocrinology, 2018,88:121–128
Leong I. Side effects of olanzapine worsened by metabolic dysfunction. Nat Rev Endocrinol, 2018,14 (3):129
Cnop M, Foufelle F, Velloso LA. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med, 2012,18(1):59–68
Lebeaupin C, Vallée D, Rousseau D, et al. Bax inhibitor-1 protects from nonalcoholic steatohepatitis by limiting inositol-requiring enzyme 1 alpha signaling in mice. Hepatology, 2018,68(2):515–532
Hu X, Zhang Q, Guo M, et al. Deletion of RNF186 expression suppresses diet-induced hepatic steatosis by regulating insulin activity. iScience, 2022,25(2):103859
Kandeil MA, Hashem RM, Mahmoud MO, et al. Zingiber officinale extract and omega-3 fatty acids ameliorate endoplasmic reticulum stress in a nonalcoholic fatty liver rat model. J Food Biochem, 2019,43(12):e13076
Ozcan L, Ergin AS, Lu A, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab, 2009,9(1):35–51
Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science, 2006,313(5790):1137–1140
Cakir I, Cyr NE, Perello M, et al. Obesity induces hypothalamic endoplasmic reticulum stress and impairs proopiomelanocortin (POMC) post-translational processing. J Biol Chem, 2013,288(24):17675–17688
Xiao Y, Deng Y, Yuan F, et al. ATF4/ATG5 Signaling in Hypothalamic Proopiomelanocortin Neurons Regulates Fat Mass via Affecting Energy Expenditure. Diabetes, 2017,66(5):1146–1158
Liu X, Zheng H. Modulation of Sirt1 and FoxO1 on Hypothalamic Leptin-Mediated Sympathetic Activation and Inflammation in Diet-Induced Obese Rats. J Am Heart Assoc, 2021,10(14):e020667
Zhang QY, Pan Y, Wang R, et al. Quercetin inhibits AMPK/TXNIP activation and reduces inflammatory lesions to improve insulin signaling defect in the hypothalamus of high fructose-fed rats. J Nutr Biochem, 2014,25(4):420–428
Yu W, Li S, Wu H, et al. Endothelial Nox4 dysfunction aggravates atherosclerosis by inducing endoplasmic reticulum stress and soluble epoxide hydrolase. Free Radic Biol Med, 2021,164:44–57
Li Y, Yang J, Chen MH, et al. Ilexgenin A inhibits endoplasmic reticulum stress and ameliorates endothelial dysfunction via suppression of TXNIP/NLRP3 inflammasome activation in an AMPK dependent manner. Pharmacol Res, 2015,99:101–115
Li W, Cao T, Luo C, et al. Crosstalk between ER stress, NLRP3 inflammasome, and inflammation. Appl Microbiol Biotechnol, 2020,104(14):6129–6140
Ko CY, Lo YM, Xu JH, et al. Alpha-lipoic acid alleviates NAFLD and triglyceride accumulation in liver via modulating hepatic NLRP3 inflammasome activation pathway in type 2 diabetic rats. Food Sci Nutr, 2021,9(5):2733–2742
Szpigel A, Hainault I, Carlier A, et al. Lipid environment induces ER stress, TXNIP expression and inflammation in immune cells of individuals with type 2 diabetes. Diabetologia, 2018,61(2):399–412
Yen IC, Tu QW, Chang TC, et al. 4-Acetylantroquinonol B ameliorates nonalcoholic steatohepatitis by suppression of ER stress and NLRP3 inflammasome activation. Biomed Pharmacother, 2021,138:111504
Zheng F, Xing S, Gong Z, et al. Silence of NLRP3 suppresses atherosclerosis and stabilizes plaques in apolipoprotein E-deficient mice. Mediators Inflamm, 2014,2014:507208
Lebeaupin C, Vallée D, Hazari Y, et al. Endoplasmic reticulum stress signalling and the pathogenesis of nonalcoholic fatty liver disease. J Hepatol, 2018,69(4):927–947
Ai Y, Sun Z, Peng C, et al. Homocysteine Induces Hepatic Steatosis Involving ER Stress Response in High Methionine Diet-Fed Mice. Nutrients, 2017,9(4):346
Varghese DS, Ali BR. Pathological Crosstalk Between Oxidized LDL and ER Stress in Human Diseases: A Comprehensive Review. Front Cell Dev Biol, 2021,9:674103
Lebeau PF, Byun JH, Platko K, et al. Diet-induced hepatic steatosis abrogates cell-surface LDLR by inducing de novo PCSK9 expression in mice. J Biol Chem, 2019,294(23):9037–9047
He M, Huang XF, Gao G, et al. Olanzapine-induced endoplasmic reticulum stress and inflammation in the hypothalamus were inhibited by an ER stress inhibitor 4-phenylbutyrate. Psychoneuroendocrinology, 2019,104:286–299
He M, Qian K, Zhang Y, et al. Olanzapine-Induced Activation of Hypothalamic Astrocytes and Toll-Like Receptor-4 Signaling via Endoplasmic Reticulum Stress Were Related to Olanzapine-Induced Weight Gain. Front Neurosci, 2020,14:589650
Albaugh VL, Judson JG, She P, et al. Olanzapine promotes fat accumulation in male rats by decreasing physical activity, repartitioning energy and increasing adipose tissue lipogenesis while impairing lipolysis. Mol Psychiatry, 2011,16(5):569–581
Weston-Green K, Huang XF, Deng C. Olanzapine treatment and metabolic dysfunction: a dose response study in female Sprague Dawley rats. Behav Brain Res, 2011,217(2):337–346
He M, Zhang Q, Deng C, et al. Hypothalamic histamine H1 receptor-AMPK signaling time-dependently mediates olanzapine-induced hyperphagia and weight gain in female rats. Psychoneuroendocrinology, 2014,42:153–164
Wiley JC, Pettan-Brewer C, Ladiges WC. Phenylbutyric acid reduces amyloid plaques and rescues cognitive behavior in AD transgenic mice. Aging Cell, 2011,10(3):418–428
de Pablo S, Rodríguez-Comas J, Díaz-Catalán D, et al. 4-Phenylbutyrate (PBA) treatment reduces hyperglycemia and islet amyloid in a mouse model of type 2 diabetes and obesity. Sci Rep, 2021,11(1):11878
He M, Yao J, Zhang Z, et al. Gold nanoclusters eliminate obesity induced by antipsychotics. Sci Rep, 2022,12(1):5502
Liu X, Wu Z, Lian J, et al. Time-dependent changes and potential mechanisms of glucose-lipid metabolic disorders associated with chronic clozapine or olanzapine treatment in rats. Sci Rep, 2017,7(1):2762
Fell MJ, Anjum N, Dickinson K, et al. The distinct effects of subchronic antipsychotic drug treatment on macronutrient selection, body weight, adiposity, and metabolism in female rats. Psychopharmacology (Berl), 2007,194(2):221–231
Victoriano M, Hermier D, Even PC, et al. Early perturbation in feeding behaviour and energy homeostasy in olanzapine-treated rats. Psychopharmacology (Berl), 2009,206(1):167–176
Zhang Q, He M, Deng C, et al. Effects of olanzapine on the elevation of macrophage infiltration and pro-inflammatory cytokine expression in female rats. J Psychopharmacol, 2014,28(12):1161–1169
Vantaggiato C, Panzeri E, Citterio A, et al. Antipsychotics Promote Metabolic Disorders Disrupting Cellular Lipid Metabolism and Trafficking. Trends Endocrinol Metab, 2019,30(3):189–210
Minokoshi Y, Toda C, Okamoto S. Regulatory role of leptin in glucose and lipid metabolism in skeletal muscle. Indian J Endocrinol Metab, 2012,16(Suppl 3):S562–S568
Verges B. Insulin sensitiviy and lipids. Diabetes Metab, 2001,27(2 Pt 2):223–227
Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in nonalcoholic fatty liver disease. Cell Mol Life Sci, 2018,75(18):3313–3327
Seo M, Islam SA, Moon SS. Acute anti-obesity effects of intracerebroventricular 11β-HSD1 inhibitor administration in diet-induced obese mice. J Neuroendocrinol, 2018,30(3):e12580
Kozuka C, Yabiku K, Sunagawa S, et al. Brown rice and its component, y-oryzanol, attenuate the preference for high-fat diet by decreasing hypothalamic endoplasmic reticulum stress in mice. Diabetes, 2012,61(12):3084–3093
Weston-Green K, Huang XF, Deng C. Alterations to melanocortinergic, GABAergic and cannabinoid neurotransmission associated with olanzapine-induced weight gain. PLoS One, 2012,7(3):e33548
Avolio E, Fazzari G, Zizza M, et al. Probiotics modify body weight together with anxiety states via pro-inflammatory factors in HFD-treated Syrian golden hamster. Behav Brain Res, 2019,356:390–399
Tang Y, Wa Q, Peng L, et al. Salvianolic Acid B Suppresses ER Stress-Induced NLRP3 Inflammasome and Pyroptosis via the AMPK/FoxO4 and Syndecan-4/Rac1 Signaling Pathways in Human Endothelial Progenitor Cells. Oxid Med Cell Longev, 2022,2022:8332825
Han CY, Rho HS, Kim A, et al. FXR Inhibits Endoplasmic Reticulum Stress-Induced NLRP3 Inflammasome in Hepatocytes and Ameliorates Liver Injury. Cell Rep, 2018,24(11):2985–2999
Fu S, Yang L, Li P, et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature, 2011,473(7348):528–531
Delis F, Rosko L, Shroff A, et al. Oral haloperidol or olanzapine intake produces distinct and region-specific increase in cannabinoid receptor levels that is prevented by high fat diet. Prog Neuropsychopharmacol Biol Psychiatry, 2017,79(Pt B):268–280
Kim J, Lee N, Suh SB, et al. Metformin ameliorates olanzapine-induced disturbances in POMC neuron number, axonal projection, and hypothalamic leptin resistance. BMB Rep, 2022,55(6):293–298
Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci, 2005,8(5):571–578
Park S, Aintablian A, Coupe B, et al. The endoplasmic reticulum stress-autophagy pathway controls hypothalamic development and energy balance regulation in leptin-deficient neonates. Nat Commun, 2020,11(1):1914
Cakir I, Nillni EA. Endoplasmic Reticulum Stress, the Hypothalamus, and Energy Balance. Trends Endocrinol Metab, 2019,30(3):163–176
Posey KA, Clegg DJ, Printz RL, et al. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab, 2009,296(5):E1003–E1012
He Y, Hara H, Nüñez G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem Sci, 2016,41(12):1012–1021
Cai M, Hu JY, Liu BB, et al. The Molecular Mechanisms of Excessive Hippocampal Endoplasmic Reticulum Stress Depressing Cognition-related Proteins Expression and the Regulatory Effects of Nrf2. Neuroscience, 2020,431:152–165
Wang NN, Zhang XX, Shen P, et al. Pinelliae rhizoma alleviated acute lung injury induced by lipopolysaccharide via suppressing endoplasmic reticulum stress-mediated NLRP3 inflammasome. Front Pharmacol, 2022,13:883865
Hou PH, Chang GR, Chen CP, et al. Long-term administration of olanzapine induces adiposity and increases hepatic fatty acid desaturation protein in female C57BL/6J mice. Iran J Basic Med Sci, 2018,21(5):495–501
Arivazhahan A, Bairy LK, Nayak V, et al. A Study to Assess the Therapeutic Effect of Enalapril on Olanzapine Induced Metabolic Syndrome in Wistar Rats. J Clin Diagn Res, 2017,11(2):FF01–FF06
Malekzadeh S, Heidari MR, Razavi BM, et al. Effect of safranal, a constituent of saffron, on olanzapine (an atypical antipsychotic) induced metabolic disorders in rat. Iran J Basic Med Sci, 2019,22(12):1476–1482
Noh DJ, Yoon GA. Mulberry (Morus alba L.) ethanol extract attenuates lipid metabolic disturbance and adipokine imbalance in high-fat fed rats. Nutr Res Pract, 2022,16(6):716–728
Lauressergues E, Bert E, Duriez P, et al. Does endoplasmic reticulum stress participate in APD-induced hepatic metabolic dysregulation? Neuropharmacology, 2012,62(2):784–796
Nissar AU, Sharma L, Mudasir MA, et al. Chemical chaperone 4-phenyl butyric acid (4-PBA) reduces hepatocellular lipid accumulation and lipotoxicity through induction of autophagy. J Lipid Res, 2017,58(9):1855–1868
Zhu Y, Guan Y, Loor JJ, et al. Fatty acid-induced endoplasmic reticulum stress promoted lipid accumulation in calf hepatocytes, and endoplasmic reticulum stress existed in the liver of severe fatty liver cows. J Dairy Sci, 2019,102(8):7359–7370
Yang G, Lee HE, Lee JY. A pharmacological inhibitor of NLRP3 inflammasome prevents non-alcoholic fatty liver disease in a mouse model induced by high fat diet. Scientific Reports, 2016,6(1):24399
Pierantonelli I, Rychlicki C, Agostinelli L, et al. Lack of NLRP3-inflammasome leads to gut-liver axis derangement, gut dysbiosis and a worsened phenotype in a mouse model of NAFLD. Sci Rep, 2017,7(1):12200
Kao AC, Spitzer S, Anthony DC, et al. Prebiotic attenuation of olanzapine-induced weight gain in rats: analysis of central and peripheral biomarkers and gut microbiota. Transl Psychiatry, 2018,8(1):66
Talukdar PM, Abdul F, Maes M, et al. A proof-of-concept study of maternal immune activation mediated induction of Toll-like receptor (TLR) and inflammasome pathways leading to neuroprogressive changes and schizophrenia-like behaviours in offspring. Eur Neuropsychopharmacol, 2021,52:48–61
Zhou R, He M, Fan J, et al. The role of hypothalamic endoplasmic reticulum stress in schizophrenia and antipsychotic-induced weight gain: A narrative review. Front Neurosci, 2022,16:947295
Kim P, Scott MR, Meador-Woodruff JH. Dysregulation of the unfolded protein response (UPR) in the dorsolateral prefrontal cortex in elderly patients with schizophrenia. Mol Psychiatry, 2021,26(4):1321–1331
Raue S, Wedekind D, Wiltfang J, et al. The Role of Proopiomelanocortin and a-Melanocyte-Stimulating Hormone in the Metabolic Syndrome in Psychiatric Disorders: A Narrative Mini-Review. Front Psychiatry, 2019,10:834
Forno F, Maatuf Y, Boukeileh S, et al. Aripiprazole Cytotoxicity Coincides with Activation of the Unfolded Protein Response in Human Hepatic Cells. J Pharmacol Exp Ther, 2020,374(3):452–461
Ozasa R, Okada T, Nadanaka S, et al. The antipsychotic olanzapine induces apoptosis in insulin-secreting pancreatic β cells by blocking PERK-mediated translational attenuation. Cell Struct Funct, 2013,38(2):183–195
Minet-Ringuet J, Even PC, Lacroix M, et al. A model for antipsychotic-induced obesity in the male rat. Psychopharmacology (Berl), 2006,187(4):447–454
Zhang X, Zhao Y, Liu Y, et al. Regulation of obesity-associated metabolic disturbance by the antipsychotic drug olanzapine: Role of the autophagy-lysosome pathway. Biochem Pharmacol, 2018,158:114–125
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
This work was supported by grants from the Natural Science Foundation of Hubei Province (No. 2021CFB301 and No. 2021CFB299), and the State Key Laboratory of Advanced Technology for Materials Synthesis and Processing (WUT) (No. 2022-KF-27).
Rights and permissions
About this article
Cite this article
Zuo, Yf., Zhang, Bh., Guo, Mr. et al. HFD-exacerbated Metabolic Side Effects of Olanzapine Are Suppressed by ER Stress Inhibitor. CURR MED SCI 43, 1116–1132 (2023). https://doi.org/10.1007/s11596-023-2781-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-023-2781-y