Abstract
Objective
Tumor-associated macrophages (TAMs) of the M2 phenotype are frequently associated with cancer progression. Invasive cancer cells undergoing epithelial-mesenchymal transition (EMT) have a selective advantage as TAM activators. Cyclin D1b is a highly oncogenic splice variant of cyclin D1. We previously reported that cyclin D1b enhances the invasiveness of breast cancer cells by inducing EMT. However, the role of cyclin D1b in inducing macrophage differentiation toward tumor-associated macrophage-like cells remains unknown. This study aimed to explore the relationship between breast cancer cells overexpressing cyclin D1b and TAMs.
Methods
Mouse breast cancer 4T1 cells were transfected with cyclin D1b variant and co-cultured with macrophage cells in a Transwell coculture system. The expression of characteristic cytokines in differentiated macrophages was detected using qRT-PCR, ELISA and zymography assay. Tumor-associated macrophage distribution in a transplanted tumor was detected by immunofluorescence staining. The proliferation and migration ability of breast cancer cells was detected using the cell counting kit-8 (CCK-8) assay, wound healing assay, Transwell invasion assay, and lung metastasis assay. Expression levels of mRNAs were detected by qRT-PCR. Protein expression levels were detected by Western blotting. The integrated analyses of The Cancer Genome Atlas (TCGA) datasets and bioinformatics methods were adopted to discover gene expression, gene coexpression, and overall survival in patients with breast cancer.
Results
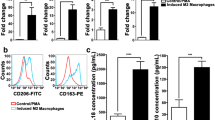
After co-culture with breast cancer cells overexpressing cyclin D1b, RAW264.7 macrophages were differentiated into an M2 phenotype. Moreover, differentiated M2-like macrophages promoted the proliferation and migration of breast cancer cells in turn. Notably, these macrophages facilitated the migration of breast cancer cells in vivo. Further investigations indicated that differentiated M2-like macrophages induced EMT of breast cancer cells accompanied with upregulation of TGF-β1 and integrin β3 expression.
Conclusion
Breast cancer cells transfected with cyclin D1b can induce the differentiation of macrophages into a tumor-associated macrophage-like phenotype, which promotes tumor metastasis in vitro and in vivo.
Similar content being viewed by others
References
Knudsen KE, Diehl JA, Haiman CA, et al. Cyclin D1: polymorphism, aberrant splicing and cancer risk. Oncogene, 2006,25(11):1620–1628
Wang Y, Dean JL, Millar EKA, et al. Cyclin D1b is aberrantly regulated in response to therapeutic challenge and promotes resistance to estrogen antagonists. Cancer Res, 2008,68(14):5628–5638
Augello MA, Berman-Booty LD, Carr3rd R, et al. Consequence of the tumor-associated conversion to cyclin D1b. EMBO Mol Med, 2015,7(5):628–647
Lu F, Gladden AB, Diehl JA. An alternatively spliced cyclin D1 isoform, cyclin D1b, is a nuclear oncogene. Cancer Res, 2003,63(21):7056–7061
Kim CJ, Tambe Y, Mukaisho K, et al. Female-specific rectal carcinogenesis in cyclin D1b transgenic mice. Carcinogenesis, 2014,35(1):227–236
Wu FH, Luo LQ, Liu Y, et al. Cyclin D1b splice variant promotes αvβ3-mediated adhesion and invasive migration of breast cancer cells. Cancer Lett, 2014,355(1):159–167
Luo BP, Luo J, Hu YB, et al. Cyclin D1b splice variant promotes αvβ3-mediated EMT induced by LPS in breast cancer cells. Curr Med Sci, 2018,38(3):467–472
Solinas G, Germano G, Mantovani A, et al. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukocyte Biol, 2009,86(5):1065–1073
Mantovani A, Sozzani S, Locati M, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol, 2002,23(11):549–555
Tang X. Tumor-associated macrophages as potential diagnostic and prognostic biomarkers in breast cancer. Cancer Lett, 2013,332(1):3–10
Ma R, Ji T, Chen D, et al. Tumor cell-derived microparticles polarize M2 tumor-associated macrophages for tumor progression. Oncoimmunology, 2016,5(4):e1118599
Wyckoff J, Wang W, Lin EY, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res, 2004,64(19):7022–7029
Rolli M, Fransvea E, Pilch J, et al. Activated integrin αvβ3 cooperates with metalloproteinase MMP-9 in regulating migration of metastatic breast cancer cells. Proc Natl Acad Sci USA, 2003,100(16):9482–9487
Hoefel D, Grooby WL, Monis PT, et al. A comparative study of carboxyfluorescein diacetate and carboxyfluorescein diacetate succinimidyl ester as indicators of bacterial activity. J Microbiol Methods, 2003,52(3):379–388
Noël W, Raes G, Ghassabeh GH, et al. Alternatively activated macrophages during parasite infections. Trends Parasitol, 2004,20(3):126–133
Peng J, Tsang JYS, Li D, et al. Inhibition of TGF-β signaling in combination with TLR7 ligation reprograms a tumoricidal phenotype in tumor-associated macrophages. Cancer Lett, 2013,331(2):239–249
Guiducci C, Vicari AP, Sangaletti S, et al. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res, 2005,65(8):3437–3446
Jouanguy E, Döffinger R, Dupuis S, et al. IL-12 and IFN-γ in host defense against mycobacteria and salmonella in mice and men. Curr Opin Immunol, 1999,11(3):346–351
Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest, 2012, 122 (3):787–795
Katara GK, Kulshrestha A, Jaiswal MK, et al. Inhibition of vacuolar ATPase subunit in tumor cells delays tumor growth by decreasing the essential macrophage population in the tumor microenvironment. Oncogene, 2016,35(8):1058–1065
Ye XZ, Xu SL, Xin YH, et al. Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-β1 signaling pathway. J Immunol, 2012,189(1):444–453
Blum A, Wang P, Zenklusen JC. SnapShot: TCGA-analyzed tumors. Cell, 2018,173(2):530
Sousa S, Brion R, Lintunen M, et al. Human breast cancer cells educate macrophages toward the M2 activation status. Breast Cancer Res, 2015,17(1):101
Kim CJ, Tambe Y, Mukaisho KI, et al. Akt-dependent activation of Erk by cyclin D1b contributes to cell invasiveness and tumorigenicity. Oncol Lett, 2016,12(6):4850–4856
Galdiero MR, Bonavita E, Barajon I, et al. Tumor associated macrophages and neutrophils in cancer. Immunobiology, 2013,218(11):1402–1410
Lorger M, Felding-Habermann B. Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am J Pathol, 2010,176(6):2958–2971
Choi J, Gyamfi J, Jang H, et al. The role of tumor-associated macrophage in breast cancer biology. Histol Histopathol, 2018,33(2):133–145
Su S, Liu Q, Chen J, et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell, 2014,25(5):605–620
Liu F, Qiu H, Xue M, et al. MSC-secreted TGF-β regulates lipopolysaccharide-stimulated macrophage M2-like polarization via the Akt/FoxO1 pathway. Stem Cell Res Ther, 2019,10(1):345
Zhang F, Wang H, Wang X, et al. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget, 2016,7(32):52294–52306
Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer, 2010,10(1):9–22
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All the authors declare that they have no competing interests.
Additional information
This study was supported by the National Natural Science Foundation of China (No. 81702920 and No. 82174020).
Supplementary data
Rights and permissions
About this article
Cite this article
Xiang, L., Rao, Q., He, B. et al. Role of Cyclin D1b in Inducing Macrophages Toward a Tumor-associated Macrophage-like Phenotype in Murine Breast Cancer. CURR MED SCI 43, 655–667 (2023). https://doi.org/10.1007/s11596-023-2762-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-023-2762-1