Abstract
Objective
This study aimed to compare the efficacy of anti-CD19 chimeric antigen receptor T cells (CAR-T cells) versus chemotherapy plus donor lymphocyte infusion (chemo-DLI) for treating relapsed CD19-positive B-cell acute lymphoblastic leukemia (B-ALL) after allogeneic hematopoietic stem cell transplantation (allo-HSCT).
Methods
Clinical data of 43 patients with B-ALL who relapsed after allo-HSCT were retrospectively analyzed. Twenty-two patients were treated with CAR-T cells (CAR-T group), and 21 with chemotherapy plus DLI (chemo-DLI group). The complete remission (CR) and minimal residual disease (MRD)-negative CR rates, leukemia-free survival (LFS) rate, overall survival (OS) rate, and incidence of acute graft-versus-host disease (aGVHD), cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) were compared between the two groups.
Results
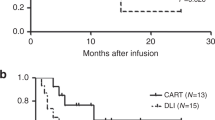
The CR and MRD-negative CR rates in the CAR-T group (77.3% and 61.5%) were significantly higher than those in the chemo-DLI group (38.1% and 23.8%) (P=0.008 and P=0.003). The 1- and 2-year LFS rates in the CAR-T group were superior to those in the chemo-DLI group: 54.5% and 50.0% vs. 9.5% and 4.8% (P=0.0001 and P=0.00004). The 1- and 2-year OS rates in the CAR-T versus chemo-DLI group were 59.1% and 54.5% vs. 19% and 9.5% (P=0.011 and P=0.003). Six patients (28.6%) with grade 2–4 aGVHD were identified in the chemo-DLI group. Two patients (9.1%) in the CAR-T group developed grade 1–2 aGVHD. Nineteen patients (86.4%) developed CRS in the CAR-T group, comprising grade 1–2 CRS in 13 patients (59.1%) and grade 3 CRS in 6 patients (27.3%). Two patients (9.1%) developed grade 1–2 ICANS.
Conclusion
Donor-derived anti-CD19 CAR-T-cell therapy may be better, safer, and more effective than chemo-DLI for B-ALL patients who relapse after allo-HSCT.
Similar content being viewed by others
References
Rosko A, Wang HL, de Lima M, et al. Reduced intensity conditioned allograft yields favorable survival for older adults with B-cell acute lymphoblastic leukemia. Am J Hematol, 2017,92(1):42–49
Shimoni A. Relapse of acute leukemia after a second allogeneic stem-cell transplantation; Is there any hope for cure? Bone Marrow Transplant, 2022,57(3):336–337
Collins RHJ, Shpilberg O, Drobyski WR, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol, 1997,15(2):433–444
Gökbuget N, Stanze D, Beck J, et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood, 2012,120(10):2032–2041
Zeidan AM, Forde PM, Symons H, et al. HLA-Haploidentical Donor Lymphocyte Infusions for Patients with Relapsed Hematologic Malignancies after Related HLA-Haploidentical Bone Marrow Transplantation. Biol Blood Marrow Transplant, 2014,20(3):314–318
Ghiso A, Raiola AM, Gualandi F, et al. DLI after haploidentical BMT with post-transplant CY. Bone Marrow Transplant, 2015,50(1):56–61
Davila ML, Brentjens RJ. CD19-Targeted CAR T cells as novel cancer immunotherapy for relapsed or refractory B-cell acute lymphoblastic leukemia. Clin Adv Hematol Oncol, 2016,14(10):802–808
Kebriaei P, Singh H, Huls MH, et al. Phase I trials using Sleeping Beauty to generate CD19-specific CAR T cells. J Clin Invest, 2016,126(9):3363–3376
Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood, 2016,127(26):3321–3330
Park JH, Geyer MB, Brentjens RJ. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood, 2016,127(26):3312–3320
Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med, 2014,371(16):1507–1517
Singh N, Perazzelli J, Grupp SA, et al. Early memory phenotypes drive T cell proliferation in patients with pediatric malignancies. Sci Transl Med, 2016,8(320):320ra323
Zhang C, Wang XQ, Zhang RL, et al. Donor-derived CD19 CAR-T cell therapy of relapse of CD19-positive B-ALL post allotransplant. Leukemia, 2021,35(6):1563–1570
Zhang C, Kong PY, Li S, et al. Donor-derived CAR-T Cells Serve as a Reduced-intensity Conditioning Regimen for Haploidentical Stem Cell Transplantation in Treatment of Relapsed/Refractory Acute Lymphoblastic Leukemia: Case Report and Review of the Literature. J Immunother, 2018,41(6):306–311
Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant, 2019,25(4):625–638
Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant, 2015,21(3):389–401
Chinese consensus on the diagnosis and management of chronic graft-versus-host disease (2021). Zhonghua Xue Ye Xue Za Zhi (Chinese), 2021,42(4):265–275
Roddie C, Peggs KS. Donor lymphocyte infusion following allogeneic hematopoietic stem cell transplantation. Expert Opin Biol Ther, 2011,11(4):473–487
Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood, 2008,112(12):4371–4383
Collins RH, Jr., Goldstein S, Giralt S, et al. Donor leukocyte infusions in acute lymphocytic leukemia. Bone Marrow Transplant, 2000,26(5):511–516
Jacoby E, Bielorai B, Avigdor A, et al. Locally produced CD19 CAR T cells leading to clinical remissions in medullary and extramedullary relapsed acute lymphoblastic leukemia. Am J Hematol, 2018,93(12):1485–1492
Park JH, Rivière I, Gonen M, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med, 2018,378(5):449–459
Chen Y, Cheng Y, Suo P, et al. Donor-derived CD19-targeted T cell infusion induces minimal residual disease-negative remission in relapsed B-cell acute lymphoblastic leukaemia with no response to donor lymphocyte infusions after haploidentical haematopoietic stem cell transplantation. Br J Haematol, 2017,179(4):598–605
Hua J, Zhang J, Zhang X, et al. Donor-derived anti-CD19 CAR T cells compared with donor lymphocyte infusion for recurrent B-ALL after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant, 2021,56(5):1056–1064
Ghosh A, Smith M, James SE, et al. Donor CD19 CAR T cells exert potent graft-versus-lymphoma activity with diminished graft-versus-host activity. Nat Med, 2017,23(2):242–249
Anwer F, Shaukat AA, Zahid U, et al. Donor origin CAR T cells: graft versus malignancy effect without GVHD, a systematic review. Immunotherapy, 2017,9(2):123–130
Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med, 2018,378(5):439–448
Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev, 2019,34:45–55
Hay KA. Cytokine release syndrome and neurotoxicity after CD19 chimeric antigen receptor-modified (CAR-) T cell therapy. Br J Haematol, 2018,183(3):364–374
Al Malki MM, Aldoss I, Stiller T, et al. Outcome of Second Allogeneic Hematopoietic Cell Transplantation in Patients With Acute Lymphoblastic Leukemia. Clin Lymphoma Myeloma and Leuk, 2016,16(9):519–522
Haen SP, Groh C, Schumm M, et al. Haploidentical hematopoietic cell transplantation using in vitro T cell depleted grafts as salvage therapy in patients with disease relapse after prior allogeneic transplantation. Ann Hematol, 2017,96(5):817–827
Nagler A, Labopin M, Dholaria B, et al. Second allogeneic stem cell transplantation in patients with acute lymphoblastic leukaemia: a study on behalf of the Acute Leukaemia Working Party of the European Society for Blood and Marrow Transplantation. Br J Haematol, 2019,186(5):767–776
Ruutu T, de Wreede LC, van Biezen A, et al. Second allogeneic transplantation for relapse of malignant disease: retrospective analysis of outcome and predictive factors by the EBMT. Bone Marrow Transplant, 2015,50(12):1542–1550
Schneidawind C, Hagmaier V, Faul C, et al. Second allogeneic hematopoietic cell transplantation enables long-term disease-free survival in relapsed acute leukemia. Ann Hematol, 2018,97(12):2491–2500
Christopeit M, Kuss O, Finke J, et al. Second allograft for hematologic relapse of acute leukemia after first allogeneic stem-cell transplantation from related and unrelated donors: the role of donor change. J Clin Oncol, 2013,31(26):3259–3271
Shah NN, Lee DW, Yates B, et al. Long-Term Follow-Up of CD19-CAR T-Cell Therapy in Children and Young Adults With B-ALL. J Clin Oncol, 2021,39(15):1650–1659
Frey NV, Shaw PA, Hexner EO, et al. Optimizing Chimeric Antigen Receptor T-Cell Therapy for Adults With Acute Lymphoblastic Leukemia. J Clin Oncol. 2020,38(5):415–422
Jiang H, Li C, Yin P, et al. Anti-CD19 chimeric antigen receptor-modified T-cell therapy bridging to allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia: An open-label pragmatic clinical trial. Am J Hematol, 2019,94(10):1113–1122
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
This work was supported by grants from the National Natural Science Foundation of China (No. 82020108004), the Hospital-level Clinical Innovation Military-Civilian Special Project of Army Medical University (No. 2018JSLC0020), Chongqing Science and Technology Innovation Leading Talent (No. CSTCCXLJRC201718), and Natural Science Foundation of Chongqing Innovation Group Science Program (No. cstc2021jcyj-cxttX0001).
Rights and permissions
About this article
Cite this article
Tan, X., Wang, Xq., Zhang, C. et al. Donor-derived CD19 CAR-T Cells versus Chemotherapy Plus Donor Lymphocyte Infusion for Treatment of Recurrent CD19-positive B-ALL After Allogeneic Hematopoietic Stem Cell Transplantation. CURR MED SCI 43, 733–740 (2023). https://doi.org/10.1007/s11596-023-2746-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-023-2746-1