Abstract
Objective
MicroRNAs are fine regulators for gene expression during the post-transcriptional stage in many autoimmune diseases. HypoxamiRs (miR-210 and miR-21) play an important role in hypoxia and in inflammation-associated hypoxia. Systemic lupus erythematosus (SLE) is a chronic systemic autoimmune disease that would potentiate many pathological complications, including hemolytic anemia. This study aimed to investigate the role of hypoxamiRs in SLE/hemolytic anemia patients.
Methods
This work was designed to analyze the circulating levels of↱ the miR-210 and miR-21 expressions and hypoxia-inducible factor-1α (HIF-α) in SLE/hemolytic anemia patients. SLE activity was evaluated for all patients by SLE Disease Activity Index (SLEDAI). Clinical manifestations/complications and serological/hematological investigations were reported. HIF-α concentration was assayed by ELISA and expression of miR-21 and miR-210 was analyzed by qRT-PCR.
Results
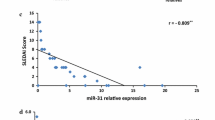
The results indicated that the fold change of the miR-210/miR-21 expressions in plasma was significantly elevated in SLE/hemolytic anemia patients. A strong positive correlation between the miR-210 and miR-21 expression levels was also recorded. Among the associated-disease complications, hypertension, arthritis, oral ulcers, and serositis were associated with a high circulating miR-210 expression, while the occurrence of renal disorders was associated with the increased miR-21 expression. Furthermore, the HIF-α level was remarkably elevated in SLE/hemolytic anemia patients. A high positive correlation was recorded between the HIF-α concentration and miR-210/miR-21 expression levels. The occurrence of oral ulcers, arthritis, and hypertension was associated with the increased HIF-α concentration. On the other hand, SLEDAI and white blood cell count were positively correlated with miR-21/ miR-210. The erythrocyte sedimentation rate was positively correlated with miR-21.
Conclusion
The dysregulation of the circulating miR-210/miR-210/HIF-1α levels in SLE/hemolytic anemia patients advocated that the hypoxia pathway might have an essential role in the pathogenesis and complications of these diseases.
Similar content being viewed by others

References
Le X, Yu X, Shen N. Novel insights of microRNAs in the development of systemic lupus erythematosus. Curr Opin Rheumatol, 2017,29(5):450–457
Banchereau R, Hong S, Cantarel B, et al. Personalized Immunomonitoring Uncovers Molecular Networks that Stratify Lupus Patients. Cell, 2016,165(3):551–565
Pacheco-Lugo L, Sáenz-García J, Navarro Quiroz E, et al. Plasma cytokines as potential biomarkers of kidney damage in patients with systemic lupus erythematosus. Lupus, 2019,28(1):34–43
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell, 2004,116(2):281–297
Ambros V. The functions of animal microRNAs. Nature, 2004,431(7006):350–355
Taganov KD, Boldin MP, Baltimore D. MicroRNAs and immunity: tiny players in a big field. Immunity, 2007,26(2):133–137
Mehta A, Baltimore D. MicroRNAs as regulatory elements in immune system logic. Nat Rev Immunol, 2016,16(5):279–294
Liu J, Qian C, Cao X. Post-Translational Modification Control of Innate Immunity. Immunity, 2016,45(1):15–30
O’Connell RM, Rao DS, Baltimore D. microRNA regulation of inflammatory responses. Annu Rev Immunol, 2012,30:295–312
Bracken CP, Scott HS, Goodall GJ. A network-biology perspective of microRNA function and dysfunction in cancer. Nat Rev Genet, 2016,17(12):719–732
Leung AK, Sharp PA. MicroRNA functions in stress responses. Mol Cell, 2010,40(2):205–215
Camps C, Buffa FM, Colella S, et al. hsa-miR-210 is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res, 2008,14(5):1340–1348
Giannakakis A, Sandaltzopoulos R, Greshock J, et al. miR-210 links hypoxia with cell cycle regulation and is deleted in human epithelial ovarian cancer. Cancer Biol Ther, 2008,7(2):255–264
Chan YC, Banerjee J, Choi SY, et al. miR-210: the master hypoxamir. Microcirculation, 2012,19(3):215–223
Huang X, Le QT, Giaccia AJ. MiR-210—micromanager of the hypoxia pathway. Trends Mol Med, 2010,16(5):230–237
Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med, 2011,364(7):656–665
Wang H, Flach H, Onizawa M, et al. Negative regulation of Hif1a expression and TH17 differentiation by the hypoxia-regulated microRNA miR-210. Nat Immunol, 2014,15(4):393–401
Dang EV, Barbi J, Yang HY, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell, 2011,146(5):772–784
Crowther M, Podolak-Dawidziak M. Hemolytic Anemia: General Considerations. McMaster Textbook of Internal Medicine. Kraków: Medycyna Praktyczna. 2020
Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum, 1982,25(11):1271–1277
Bombardier C, Gladman DD, Urowitz MB, et al. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum, 1992,35(6):630–640
Chun HY, Chung JW, Kim HA, et al. Cytokine IL-6 and IL-10 as biomarkers in systemic lupus erythematosus. J Clin Immunol, 2007,27(5):461–466
Mok MY, Wu HJ, Lo Y, et al. The relation of interleukin 17 (IL-17) and IL-23 to Th1/Th2 cytokines and disease activity in systemic lupus erythematosus. J Rheumatol, 2010,37(10):2046–2052
World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA, 2013,310(20):2191–2194
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods, 2001,25(4):402–408
Wu YH, Chan YF, Hsieh HL, et al. Upregulation of miR-210-5p in Systemic Lupus Erythematosus Impairs Silent Clearance of Dead Cell Remnants. FASEB J, 2020,34(S1), Supplement: Experimental Biology, Meeting Abstracts, Pages 1–1.
Huang Q, Chen SS, Li J, et al. miR-210 expression in PBMCs from patients with systemic lupus erythematosus and rheumatoid arthritis. Ir J Med Sci, 2018,187(1):243–249
Dai Y, Huang YS, Tang M, et al. Microarray analysis of microRNA expression in peripheral blood cells of systemic lupus erythematosus patients. Lupus, 2007,16(12):939–946
Higgins DF, Kimura K, Bernhardt WM, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest, 2007,117(12):3810–3820
Kimura K, Iwano M, Higgins DF, et al. Stable expression of HIF-1alpha in tubular epithelial cells promotes interstitial fibrosis. Am J Physiol Renal Physiol, 2008,295(4):F1023–1029
Ma C, Wei J, Zhan F, et al. Urinary hypoxia-inducible factor-1alpha levels are associated with histologic chronicity changes and renal function in patients with lupus nephritis. Yonsei Med J, 2012,53(3):587–592
Deng W, Ren Y, Feng X, et al. Hypoxia inducible factor-1 alpha promotes mesangial cell proliferation in lupus nephritis. Am J Nephrol, 2014,40(6):507–515
Garchow B, Maque Acosta Y, et al. HIF-1α and miR-210 differential and lineage-specific expression in systemic lupus erythematosus. Mol Immunol, 2021,133:128–134
Rigó J, Molvarec A, Nagy B, et al. Expression analysis of circulating exosomal hsa-miR-210 in hypertensive disorders of pregnancy: Biomarkers, prediction of preeclampsia. Pregnancy Hypertens, 2016,6(3):183
Acknowledgment
This work was supported by the Taif University Researchers Supporting Project (No. TURSP-2020/103).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Gamal-Eldeen, A.M., Fahmy, C.A., Raafat, B.M. et al. Circulating Levels of Hypoxia-regulating MicroRNAs in Systemic Lupus Erythematosus Patients with Hemolytic Anemia. CURR MED SCI 42, 1231–1239 (2022). https://doi.org/10.1007/s11596-022-2644-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-022-2644-y