Abstract
Objective
Follistatin (FST) inhibits the action of activin by interfering with the binding of activin to its receptor. Although the prognostic value of FST in various cancers has been investigated previously, studies rarely focused on hypopharyngeal carcinoma (HPC). In our study, the effect of FST expression on HPC tissues and cell lines was investigated.
Methods
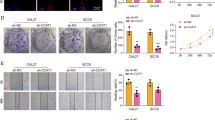
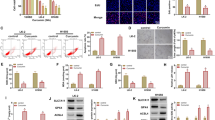
A total of 60 patients with HPC were recruited for this study. Levels of FST mRNA and protein were measured by quantitative polymerase chain reaction (PCR) and immunohistochemistry in HPC tissue samples and by qPCR in the HPC FaDu cells, as well as immortal nasopharyngeal epithelial cell line NP-69 cells. After silencing the FST expression in FaDu cells using lentivirus-mediated siRNA that was specific for FST mRNA, cell proliferation was determined by a Celigo assay. Tumor growth was monitored in nude mice and viability was determined by a methylthiazoletetrazolium assay. The ratio of cell cycle arrest and apoptosis was evaluated by flow cytometry. The colony formation ability was performed using Giemsa staining. In addition, wound healing and Transwell migration and invasion assays were performed for the analysis of cell motility.
Results
FST expression was significantly higher in human HPC tissue and FaDu cells than in normal tissue and NP-69 cells. A higher expression of FST in HPC samples was positively correlated with advanced tumors. Moreover, FST knockdown by shRNA significantly decreased cell growth, colony formation, migration and invasion. Furthermore, FST silencing increased the cell apoptosis percentage, and arrested cell cycle in the S phase in FaDu cells. In addition, FST silencing suppressed tumor growth in vivo.
Conclusions
Our results indicated that the FST gene was associated with HPC progression and may serve as a potential therapeutic target for the treatment of HPC.
Similar content being viewed by others
References
Gooi Z, Fakhry C, Goldenberg D, et al. AHNS Series: Do you know your guidelines? Principles of radiation therapy for head and neck cancer: A review of the National Comprehensive Cancer Network guidelines. Head Neck, 2016,38(7):987–992
Ye LL, Rao J, Fan XW, et al. Impact of tumor dimensions and lymph node density on the survival of patients with hypopharyngeal squamous cell carcinoma. Cancer Manag Res, 2018,10:4679–4688
Tian J, Liu X, Liu X, et al. Notch1 serves as a prognostic factor and regulates metastasis via regulating EGFR expression in hypopharyngeal squamous cell carcinoma. Onco Targets Ther, 2018,11:7395–7405
Han Y, Zhang M, Chen D, et al. Downregulation of RNA binding motif protein 17 expression inhibits proliferation of hypopharyngeal carcinoma FaDu cells. Oncol Lett, 2018,15(4):5680–5684
Sepporta MV, Tumminello FM, Flandina C, et al. Follistatin as potential therapeutic target in prostate cancer. Target Oncol, 2013,8(4):215–223
Sugino K, Kurosawa N, Nakamura T, et al. Molecular heterogeneity of follistatin, an activin-binding protein. Higher affinity of the carboxyl-terminal truncated forms for heparan sulfate proteoglycans on the ovarian granulosa cell. J Biol Chem, 1993,268(21):15579–15587
Phillips DJ, De Kretser DM. Follistatin: a multifunctional regulatory protein. Front Neuroendocrinol, 1998,19(4): 287–322
Thompson TB, Lerch TF, Cook RW, et al. The structure of the follistatin:activin complex reveals antagonism of both type I and type II receptor binding. Dev Cell, 2005,9(4):535–543
Sebastiani G, Díaz M, López-Bermejo A, et al. Circulating follistatin in the human foetus at term birth. Pediatr Obes, 2012,7(1):39–43
Welt CK. The physiology and pathophysiology of inhibin, activin and follistatin in female reproduction. Curr Opin Obstet Gynecol, 2002,14(3):317–323
Bartholin L, Maguer-Satta V, Hayette S, et al. Transcription activation of FLRG and follistatin by activin A, through Smad proteins, participates in a negative feedback loop to modulate activin A function. Oncogene, 2002,21(14):2227–2235
Singh R, Bhasin S, Braga M, et al. Regulation of myogenic differentiation by androgens: cross talk between androgen receptor/beta-catenin and follistatin/transforming growth factor-beta signaling pathways. Endocrinology, 2009,150(3):1259–1268
Winters SJ, Ghooray D, Fujii Y, et al. Transcriptional regulation of follistatin expression by GnRH in mouse gonadotroph cell lines: evidence for a role for cAMP signaling. Mol Cell Endocrinol, 2007,271(1–2):45–54
Necela BM, Su W, Thompson EA. Peroxisome proliferator-activated receptor gamma down-regulates follistatin in intestinal epithelial cells through SP1. J Biol Chem, 2008,283(44):29784–29794
Kang W, Saqui-Salces M, Zavros Y, et al. Induction of follistatin precedes gastric transformation in gastrin deficient mice. Biochem Biophys Res Commun, 2008,376(3):573–577
Eichberger T, Sander V, Schnidar H, et al. Overlapping and distinct transcriptional regulator properties of the GLI1 and GLI2 oncogenes. Genomics, 2006,87(5):616–632
Harkonen P, Torn S, Kurkela R, et al. Sex hormone metabolism in prostate cancer cells during transition to an androgen-independent state. J Clin Endocrinol Metab, 2003,88(2):705–712
Rossmanith W, Chabicovsky M, Grasl-Kraupp B, et al. Follistatin overexpression in rodent liver tumors: a possible mechanism to overcome activin growth control. Mol Carcinog, 2002,35(1):1–5
van Schaik RH, Wierikx CD, Timmerman MA, et al. Variations in activin receptor, inhibin/activin subunit and follistatin mRNAs in human prostate tumour tissues. Br J Cancer, 2000,82(1):112–117
Simone ND, Crowley Jr WF, Wang QF, et al. Characterization of inhibin/activin subunit, follistatin, and activin type II receptors in human ovarian cancer cell lines: a potential role in autocrine growth regulation. Endocrinology, 1996,137(2):486–494
Shikone T, Matzuk MM, Perlas E, et al. Characterization of gonadal sex cord-stromal tumor cell lines from inhibin-alpha and p53-deficient mice: the role of activin as an autocrine growth factor. Mol Endocrinol, 1994,8(8):983–995
Seachrist DD, Sizemore ST, Johnson E, et al. Follistatin is a metastasis suppressor in a mouse model of HER2-positive breast cancer. Breast Cancer Res, 2017,19(1):66
Zabkiewicz C, Resaul J, Hargest R, et al. Increased Expression of Follistatin in Breast Cancer Reduces Invasiveness and Clinically Correlates with Better Survival. Cancer Genomics Proteomics, 2017,14(4):241–251
Schneider CA, Rasband WS, Eliceiri K W. NIH Image to ImageJ: 25 years of image analysis. Nat Methods, 2012,9(7):671–675
Ren P, Chen FF, Liu HY, et al. High serum levels of follistatin in patients with ovarian cancer. J Int Med Res, 2012,40(3):877–886
Tomoda T, Nouso K, Miyahara K, et al. Prognotic impact of serum follistatin in patients with hepatocellular carcinoma. J Gastroenterol Hepatol, 2013,28(8):1391–1396
Ogino H, Yano S, Kakiuchi S, et al. Follistatin suppresses the production of experimental multiple-organ metastasis by small cell lung cancer cells in natural killer cell-depleted SCID mice. Clin Cancer Res, 2008,14(3):660–667
Zhang P, Ruan Y, Xiao J, et al. Association of serum follistatin levels with histological types and progression of tumor in human lung cancer. Cancer Cell Int, 2018,18:162
Tumminello FM, Badalamenti G, Fulfaro F, et al. Serum follistatin in patients with prostate cancer metastatic to the bone. Clin Exp Metastasis, 2010,27(8):549–555
Leto G, Incorvaia L, Flandina C, et al. Clinical Impact of Cystatin C/Cathepsin L and Follistatin/Activin A Systems in Breast Cancer Progression: A Preliminary Report. Cancer Invest, 2016,34(9):415–423
Bucher N, Britten CD. G2 checkpoint abrogation and checkpoint kinase-1 targeting in the treatment of cancer. Br J Cancer, 2008,98(3):523–528
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that there is no conflict of interest with any financial organization or corporation or individual that can inappropriately influence this work.
Additional information
This study was supported by the Project of Young and Middle-aged Scientific Research Fund of Wannan Medical College (No. WK2019F11).
Rights and permissions
About this article
Cite this article
Ge, L., Liu, Sf. Lentivirus-Mediated Short Hairpin RNA for Follistatin Downregulation Suppresses Tumor Progression in Hypopharyngeal Carcinoma. CURR MED SCI 42, 832–840 (2022). https://doi.org/10.1007/s11596-022-2615-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-022-2615-3