Abstract
Objective
Methotrexate (MTX) can be safely administered to most patients but may cause severe toxicity in others. This study aimed to summarize the characteristics of high-dose methotrexate (HD-MTX) chemotherapy and to evaluate whether the modified dose-adjustment program was able to improve the maintenance of sufficient MTX exposure levels while minimizing toxicities.
Methods
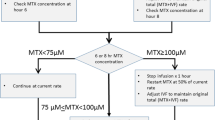
We evaluated 1172 cycles of high-dose MTX chemotherapy from 294 patients who were treated according to the CCCG-ALL-2015 protocol (clinical trial number: ChiCTR-IPR-14005706) and analyzed the data of actual MTX dosage, MTX concentration, toxicity, and prognosis. We compared data between the dose-adjustment Program 1 (fixed 20% reduction in dose) and the dose-adjustment Program 2 (dose-individualization based on reassessment of the creatine clearance rate and the MTX concentration-monitoring point at 16 h), which were applied if the MTX clearance was delayed in the previous cycle.
Results
The patients who used Program 2 had higher actual MTX infusion doses and infusion rates and were able to better maintain the MTX concentration at 44 h at the established target value than those on Program 1 (P<0.001). No significant differences in toxicities were found between these two programs except that abnormal serum potassium levels and prolonged myelosuppression in intermediate-risk/high-risk patients were more frequently observed in patients using Program 2 (P<0.001). No significant correlations were observed between the MTX dose, dose-adjustment programs, or MTX concentrations and relapse-free survival.
Conclusion
Adjusting the MTX dose using Program 2 is more efficient for maintaining sufficient MTX exposure without significantly increasing the toxicity.
Similar content being viewed by others
References
Wijaya J, Gose T, Schuetz JD. Using pharmacology to squeeze the life out of childhood leukemia, and potential strategies to achieve breakthroughs in medulloblastoma treatment. Pharmacol Rev, 2020,72(3):668–691
Moricke A, Zimmermann M, Valsecchi MG, et al. Dexamethasone vs prednisone in induction treatment of pediatric all: Results of the randomized trial aieop-bfm all 2000. Blood, 2016,127(17):2101–2112
Howard SC, McCormick J, Pui CH, et al. Preventing and managing toxicities of high-dose methotrexate. Oncologist, 2016,21(12):1471–1482
Pui CH, Campana D, Pei DQ, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med, 2009,360(26):2730–2741
Pui CH, Sandlund JT, Pei D, et al. Improved outcome for children with acute lymphoblastic leukemia: Results of total therapy study xiiib at st jude children’s research hospital. Blood, 2004,104(9):2690–2696
Levêque D, Becker G, Toussaint E, et al. Clinical pharmacokinetics of methotrexate in oncology. Int J Pharmacokinet, 2017,2(2):137–147
Evans WE, Crom WR, Abromowitch M, et al. Clinical pharmacodynamics of high-dose methotrexate in acute lymphocytic leukemia. Identification of a relation between concentration and effect. N Engl J Med, 1986,314(8):471–477
Foster JH, Thompson PA, Bernhardt MB, et al. A prospective study of a simple algorithm to individually dose high-dose methotrexate for children with leukemia at risk for methotrexate toxicities. Cancer Chemother Pharmacol, 2019,83(2):349–360
Pauley JL, Panetta JC, Crews KR, et al. Between-course targeting of methotrexate exposure using pharmacokinetically guided dosage adjustments. Cancer Chemother Pharmacol, 2013,72(2):369–378
Wall AM, Gajjar A, Link A, et al. Individualized methotrexate dosing in children with relapsed acute lymphoblastic leukemia. Leukemia, 2000,14(2):221–225
Foster JH, Bernhardt MB, Thompson PA, et al. Using a bedside algorithm to individually dose high-dose methotrexate for patients at risk for toxicity. J Pediatr Hematol Oncol, 2017,39(1):72–76
Kawakatsu S, Nikanjam M, Lin M, et al. Population pharmacokinetic analysis of high-dose methotrexate in pediatric and adult oncology patients. Cancer Chemother Pharmacol, 2019,84(6):1339–1348
Institute NC. Common terminology criteria for adverse events (ctcae) version 5.0. National Cancer Institute. 27 November, 2017. Available at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Accessed 10 September, 2020
Abromowitch M, Ochs J, Pui CH, et al. High-dose methotrexate improves clinical outcome in children with acute lymphoblastic leukemia: St. Jude total therapy study x. Med Pediatr Oncol, 1988,16(5):297–303
Ishizaki J, Nakano C, Kitagawa K, et al. A previously unknown drug-drug interaction is suspected in delayed elimination of plasma methotrexate in high-dose methotrexate therapy. Ann Pharmacother, 2020,54(1):29–35
Stoller RG, Hande KR, Jacobs SA, et al. Use of plasma pharmacokinetics to predict and prevent methotrexate toxicity. N Engl J Med, 1977,297(12):630–634
Rivera GK, Raimondi SC, Hancock ML, et al. Improved outcome in childhood acute lymphoblastic leukaemia with reinforced early treatment and rotational combination chemotherapy. Lancet, 1991,337(8733):61–66
Green DM, Brecher ML, Blumenson LE, et al. The use of intermediate dose methotrexate in increased risk childhood acute lymphoblastic leukemia. A comparison of three versus six courses. Cancer, 1982,50(12):2722–2727
Pui CH, Pei D, Campana D, et al. Improved prognosis for older adolescents with acute lymphoblastic leukemia. J Clin Oncol, 2011,29(4):386–391
Evans WE, Relling MV, Rodman JH, et al. Conventional compared with individualized chemotherapy for childhood acute lymphoblastic leukemia. N Engl J Med, 1998,338(8):499–505
Synold TW, Relling MV, Boyett JM, et al. Blast cell methotrexate-polyglutamate accumulation in vivo differs by lineage, ploidy, and methotrexate dose in acute lymphoblastic leukemia. J Clin Invest, 1994,94(5):1996–2001
Reiter A, Schrappe M, Ludwig WD, et al. Chemotherapy in 998 unselected childhood acute lymphoblastic leukemia patients. Results and conclusions of the multicenter trial all-bfm 86. Blood, 1994,84(9):3122–3133
Pui CH, Sallan S, Relling MV, et al. International childhood acute lymphoblastic leukemia workshop: Sausalito, ca, 30 november-1 december 2000. Leukemia, 2001,15(5):707–715
Tsurusawa M, Gosho M, Mori T, et al. Statistical analysis of relation between plasma methotrexate concentration and toxicity in high-dose methotrexate therapy of childhood nonhodgkin lymphoma. Pediatr Blood Cancer, 2015,62(2):279–284
Wang X, Song YQ, Wang JJ, et al. Effect of proton pump inhibitors on high-dose methotrexate elimination: A systematic review and meta-analysis. Int J Clin Pharm, 2020,42(1):23–30
Cwiklinska M, Czogala M, Kwiecinska K, et al. Polymorphisms of slc19a1 80 g>a, mthfr 677 c>t, and tandem ts repeats influence pharmacokinetics, acute liver toxicity, and vomiting in children with acute lymphoblastic leukemia treated with high doses of methotrexate. Front Pediatri, 2020,8:307
Tiwari P, Thomas MK, Pathania S, et al. Serum creatinine versus plasma methotrexate levels to predict toxicities in children receiving high-dose methotrexate. Pediatr Hematol Oncol, 2015,32(8):576–584
Salzer WL, Winick NJ, Wacker P, et al. Plasma methotrexate, red blood cell methotrexate, and red blood cell folate values and outcome in children with precursor b-acute lymphoblastic leukemia: A report from the children’s oncology group. J Pediatr Hematol Oncol, 2012,34(1):e1–7
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Run-ming JIN is a member of the Editorial Board for Current Medical Science. Author Fen ZHOU is a member of Youth Editorial Committee for Current Medical Science. The paper was handled by other editors and has undergone rigorous peer review process. Authors Run-ming JIN and Fen ZHOU were not involved in the journal’s review of, or decisions related to, this manuscript.
Additional information
The present study was supported by the National Natural Science Foundation of China (No. 81700147 and No. 82070172).
Rights and permissions
About this article
Cite this article
Shen, Yq., Wang, Zj., Wu, Xy. et al. Dose-individualization Efficiently Maintains Sufficient Exposure to Methotrexate without Additional Toxicity in High-dose Methotrexate Regimens for Pediatric Acute Lymphoblastic Leukemia. CURR MED SCI 42, 769–777 (2022). https://doi.org/10.1007/s11596-022-2589-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-022-2589-1