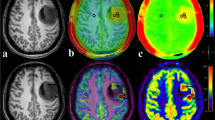
Abstract
Objective
Isocitrate dehydrogenase gene (IDH) mutations are associated with tumor angiogenesis and therefore play an important role in glioma management. This study compared the performance of tumor blood vessels counted from contrast-enhanced 3D brain volume (3D-BRAVO) sequence and dynamic contrast-enhanced (DCE) MRI in differentiating IDH1 status in gliomas.
Methods
Forty-four glioma patients [16 with IDH1 mutant-type (IDH1-MT), 28 with IDH1 wild-type (IDH1-WT)] were retrospectively analyzed. A blood vessel entering a tumor was defined as an intratumoral vessel; a blood vessel adjacent to the edge of a tumor was defined as a peritumoral vessel. Combined vessels were defined as the sum of the intratumoral and peritumoral vessels. DCE-derived metrics of tumor were normalized to the contralateral normal-appearing white matter.
Results
Intratumoral, peritumoral, and combined tumor blood vessels were all significantly different between IDH1-MT and IDH1-WT gliomas, and the range of area under curves (AUCs) was 0.816–0.855. For DCE-derived parameters, cerebral blood volume, cerebral blood flow, mean transit time, and volume transfer constant were significantly different between IDH1-MT and IDH1-WT gliomas, and the range of AUCs was 0.703–0.756. Combined vessels possessed the best performance for identifying IDH1 mutations in gliomas (AUC: 0.855, sensitivity: 0.857, specificity: 0.812, P<0.001).
Conclusion
The number of tumor blood vessels has comparable diagnostic performance with DCE-derived parameters for differentiating IDH1 mutations and can serve as a potential imaging biomarker to reflect IDH1 mutations in gliomas.
Similar content being viewed by others
References
Bello L, Giussani C, Carrabba G, et al. Angiogenesis and invasion in gliomas. Cancer Treat Res, 2004,117:263–284
Burger PC, Vogel FS, Green SB, et al. Glioblastoma multiforme and anaplastic astrocytoma. Pathologic criteria and prognostic implications. Cancer, 1985,56 (5):1106–1111
Daumas-Duport C, Tucker ML, Kolles H, et al. Oligodendrogliomas. Part II: A new grading system based on morphological and imaging criteria. J Neurooncol, 1997,34(1):61–78
Abdulrauf SI, Edvardsen K, Ho KL, et al. Vascular endothelial growth factor expression and vascular density as prognostic markers of survival in patients with low-grade astrocytoma. J Neurosurg, 1998,88(3):513–520
Hardee ME, Zagzag D. Mechanisms of glioma-associated neovascularization. Am J Pathol, 2012,181 (4):1126–1141
Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathologica, 2016,131(6):803–820
Lai A, Kharbanda S, Pope WB, et al. Evidence for sequenced molecular evolution of IDH1 mutant glioblastoma from a distinct cell of origin. J Clin Oncol, 2011,29(34):4482–4490
Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med, 2009,360(8):765–773
Balss J, Meyer J, Mueller W, et al. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol, 2008,116(6):597–602
Dang L, White D W, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature, 2009,462(7274):739–744
Ye D, Ma S, Xiong Y, et al. R-2-hydroxyglutarate as the key effector of IDH mutations promoting oncogenesis. Cancer Cell, 2013,23(3):274–276
Koivunen P, Lee S, Duncan CG, et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature, 2012,483(7390):484–488
Kickingereder P, Sahm F, Radbruch A, et al. IDH mutation status is associated with a distinct hypoxia/angiogenesis transcriptome signature which is non-invasively predictable with rCBV imaging in human glioma. Sci Rep, 2015,5:16238
Turkalp Z, Karamchandani J, Das S. IDH mutation in glioma: new insights and promises for the future. JAMA Neurol, 2014,71(10):1319–1325
Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol, 2009,27(28):4733–4740
Tovi M, Lilja A, Bergstrom M, et al. Delineation of gliomas with magnetic resonance imaging using Gd-DTPA in comparison with computed tomography and positron emission tomography. Acta Radiol, 1990,31(5):417–429
Jahng GH, Li KL, Ostergaard L, et al. Perfusion magnetic resonance imaging: a comprehensive update on principles and techniques. Korean J Radiol, 2014, 15(5):554–577
Brendle C, Hempel JM, Schittenhelm J, et al. Glioma Grading and Determination of IDH Mutation Status and ATRX loss by DCE and ASL Perfusion. Clin Neuroradiol, 2018,28(3):421–428
You SH, Choi SH, Kim TM, et al. Differentiation of High-Grade from Low-Grade Astrocytoma: Improvement in Diagnostic Accuracy and Reliability of Pharmacokinetic Parameters from DCE MR Imaging by Using Arterial Input Functions Obtained from DSC MR Imaging. Radiology, 2018,286(3):981–991
Patel SH, Poisson LM, Brat DJ, et al. T2—FLAIR Mismatch, an Imaging Biomarker for IDH and 1p/19q Status in Lower-grade Gliomas: A TCGA/TCIA Project. Clin Cancer Res, 2017,23(20):6078–6085
Xi Y, Kang X, Wang N, et al. Differentiation of primary central nervous system lymphoma from high-grade glioma and brain metastasis using arterial spin labeling and dynamic contrast-enhanced magnetic resonance imaging. Eur J Radiol, 2019,112:59–64
Tofts PS. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging, 1997,7(1):91–101
Kellner E, Breyer T, Gall P, et al. MR evaluation of vessel size imaging of human gliomas: Validation by histopathology. J Magn Reson Imaging, 2015,42(4): 1117–1125
Lemasson B, Valable S, Farion R, et al. In vivo imaging of vessel diameter, size, and density: a comparative study between MRI and histology. Magn Reson Med, 2013,69(1):18–26
Kiselev V G, Strecker R, Ziyeh S, et al. Vessel size imaging in humans. Magn Reson Med, 2005,53(3):553–563
Tropres I, Grimault S, Vaeth A, et al. Vessel size imaging. Magn Reson Med, 2001,45(3):397–408
Guo H, Kang H, Tong H, et al. Microvascular characteristics of lower-grade diffuse gliomas: investigating vessel size imaging for differentiating grades and subtypes. Eur Radiol, 2019,29(4):1893–1902
Stadlbauer A, Zimmermann M, Kitzwogerer M, et al. MR Imaging-derived Oxygen Metabolism and Neovascularization Characterization for Grading and IDH Gene Mutation Detection of Gliomas. Radiology, 2017,283(3):799–809
Kleihues P, Soylemezoglu F, Schauble B, et al. Histopathology, classification, and grading of gliomas. Glia, 1995,15(3):211–221
Jafari-Khouzani K, Loebel F, Bogner W, et al. Volumetric relationship between 2-hydroxyglutarate and FLAIR hyperintensity has potential implications for radiotherapy planning of mutant IDH glioma patients. Neuro Oncol, 2016,18(11):1569–1578
Mur P, Mollejo M, Hernández-Iglesias T, et al. Molecular Classification Defines 4 Prognostically Distinct Glioma Groups Irrespective of Diagnosis and Grade. J Neuropathol Exp Neurol, 2015,74(3):241–249
Beiko J, Suki D, Hess K R, et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol, 2014,16(1):81–91
Pellegatta S, Valletta L, Corbetta C, et al. Effective immuno-targeting of the IDH1 mutation R132H in a murine model of intracranial glioma. Acta Neuropathol Commun, 2015,3:4
Rohle D, Popovici-Muller J, Palaskas N, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science, 2013,340(6132):626–630
Turcan S, Fabius AW, Borodovsky A, et al. Efficient induction of differentiation and growth inhibition in IDH1 mutant glioma cells by the DNMT Inhibitor Decitabine. Oncotarget, 2013,4(10):1729–1736
Gomez-Manzano C, Holash J, Fueyo J, et al. VEGF Trap induces antiglioma effect at different stages of disease. Neuro Oncol, 2008,10(6):940–945
Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A, 2002,99(17):11393–11398
Cortes-Santiago N, Hossain MB, Gabrusiewicz K, et al. Soluble Tie2 overrides the heightened invasion induced by anti-angiogenesis therapies in gliomas. Oncotarget, 2016,7(13):16146–16157
Huang H, Bhat A, Woodnutt G, et al. Targeting the ANGPT-TIE2 pathway in malignancy. Nat Rev Cancer, 2010,10(8):575–585
Chae SS, Kamoun WS, Farrar CT, et al. Angiopoietin-2 interferes with anti-VEGFR2-induced vessel normalization and survival benefit in mice bearing gliomas. Clin Cancer Res, 2010,16(14):3618–3627
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interest Statement
The authors declare that they have no conflicts of interest.
Author Wen-zhen ZHU is a member of the Editorial Board for [Current Medical Science]. The paper was handled by other editors and has undergone rigorous peer review process. Author Wen-zhen ZHU was not involved in the journal’s review of, or decisions related to, this manuscript.
This study was supported by the National Natural Science Foundation of China (No. 81730049 and No. 81801666) and the Fundamental Research Funds for the Central Universities, HUST (No. 2019JYCXJJ044).
Rights and permissions
About this article
Cite this article
Li, Sh., Shen, Nx., Wu, D. et al. A Comparative Study Between Tumor Blood Vessels and Dynamic Contrast-enhanced MRI for Identifying Isocitrate Dehydrogenase Gene 1 (IDH1) Mutation Status in Glioma. CURR MED SCI 42, 650–657 (2022). https://doi.org/10.1007/s11596-022-2563-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-022-2563-y