Abstract
Objective
At present, a number of very severe aplastic anemia (VSAA) patients cannot receive hematopoietic stem cell transplantation (HSCT) or standard immunosuppressive therapy (IST) due to the high cost of therapy, shortage of sibling donors, and lack of resources to support the HSCT. In addition, some VSAA patients with autoantibodies have no life-threatening infections or bleeding at the time of initial diagnosis. Considering the disease condition, economics and other factors, the present study designed a new and relatively mild treatment strategy: cyclosporine A plus pulsed high-dose prednisone (CsA+HDP).
Methods
The present study retrospectively analyzed 11 VSAA patients, who were treated with CsA+HDP in our hospital from August 2017 to August 2019.
Results
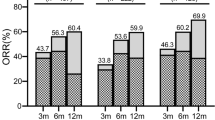
The median follow-up time for these patients was 24.9 months. The overall response rate was 54.5% (6/11) at six months after the initiation of IST and 81.8% (9/11) at deadline. Five patients achieved complete remission and four patients met the criteria for partial response at the last follow-up. The median time to response for responders was 110 days. Three patients underwent HSCT due to the poor effect of CsA+HDP or to find a suitable transplant donor. Recurrence and clonal evolution were not found in any of these patients. The estimated 3-year overall survival rate and 3-year failure-free survival rate were 100.0% and 72.7%, respectively. In addition, the results revealed that the cyclosporine-prednisone-associated toxicity was mild and well-tolerated by most patients.
Conclusion
The novel CsA+HDP regimen has good therapeutic effect and safety for VSAA patients with autoantibodies, who have no serious life-threatening infections or bleeding at the time of initial diagnosis.
Similar content being viewed by others
References
Mary JY, Baumelou E, Guiguet M. Epidemiology of aplastic anemia in France: a prospective multicentric study. The French Cooperative Group for Epidemiological Study of Aplastic Anemia. Blood, 1990,75(8):1646–1653
Montané E, Ibáñez L, Vidal X, et al. Epidemiology of aplastic anemia: a prospective multicenter study. Haematologica, 2008,93(4):518–523
Wang L, Liu H. Pathogenesis of aplastic anemia. Hematology, 2019,24(1):559–566
Zeng Y, Katsanis E. The complex pathophysiology of acquired aplastic anaemia. Clin Exp Immunol, 2015, 180(3):361–370
Young NS. Aplastic Anemia. N Engl J Med, 2018,379(17):1643–1656
Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol, 2016,172(2):187–207
Yoshida N, Kobayashi R, Yabe H, et al. First-line treatment for severe aplastic anemia in children:bone marrow transplantation from a matched family donor versus immunosuppressive therapy. Haematologica, 2014,99(12):1784–1791
Dufour C, Pillon M, Socie G, et al. Outcome of aplastic anaemia in children. A study by the severe aplastic anaemia and paediatric disease working parties of the European group blood and bone marrow transplant. Br J Haematol, 2015,169(4):565–573
Rogers ZR, Nakano TA, Olson TS, et al. Immunosuppressive therapy for pediatric aplastic anemia: a North American Pediatric Aplastic Anemia Consortium study. Haematologica. 2019,104(10):1974–1983
Zhu XF, He HL, Wang SQ, et al. Current Treatment Patterns of Aplastic Anemia in China: A Prospective Cohort Registry Study. Acta Haematol, 2019,142(3): 162–170
Mahapatra M, Singh PK, Agarwal M, et al. Epidemiology, Clinico-Haematological Profile and Management of Aplastic Anaemia: AIIMS Experience. J Assoc Physicians India, 2015,63(3 Suppl):30–35
Pierri F, Dufour C. Management of aplastic anemia after failure of frontline immunosuppression. Expert Rev Hematol, 2019,12(10):809–819
Kosaka Y, Yagasaki H, Sano K, et al. Prospective multicenter trial comparing repeated immunosuppressive therapy with stem-cell transplantation from an alternative donor as second line treatment for children with severe and very severe aplastic anemia. Blood, 2008,111(3):1054–1059
Peffault de Latour R, Chevret S, Jubert C, et al. Unrelated cord blood transplantation in patients with idiopathic refractory severe aplastic anemia: a nationwide phase 2 study. Blood, 2018,132(7):750–754
Scheinberg P, Nunez O, Weinstein B, et al. Activity of alemtuzumab monotherapy in treatment-naive, relapsed, and refractory severe acquired aplastic anemia. Blood, 2012,119(2):345–354
Brodsky RA, Chen AR, Dorr D, et al. High-dose cyclophosphamide for severe aplastic anemia: long-term follow-up. Blood, 2010,115(11):2136–2141
Kook H, Chung NG, Kang HJ, et al. Acquired aplastic anemia in Korean children: treatment guidelines from the Bone Marrow Failure Committee of the Korean Society of Pediatric Hematology Oncology. Int J Hematol, 2016,103(4):380–386
Yoshida N, Kojima S. Updated Guidelines for the Treatment of Acquired Aplastic Anemia in Children. Curr Oncol Rep, 2018,20(9):67
Assi R, Garcia-Manero G, Ravandi F. Addition of eltrombopag to immunosuppressive therapy in patients with newly diagnosed aplastic anemia. Cancer, 2018,124(21):4192–4201
Cooper N, Ghanima W. Immune Thrombocytopenia. N Engl J Med, 2019,381(10):945–955
Audia S, Mahévas M, Samson M, et al. Pathogenesis of immune thrombocytopenia. Autoimmun Rev, 2017, 16(6):620–632
Zufferey A, Kapur R, Semple JW. Pathogenesis and Therapeutic Mechanisms in Immune Thrombocytopenia (ITP). J Clin Med, 2017,6(2):16
Liu Q, Xu H, Guan X, et al. Clinical Significance of Antinuclear and Antiextractable Nuclear Antigen Antibody in Childhood Immune Thrombocytopenia. Semin Thromb Hemost, 2017,43(6):629–634
Bidot CJ, Jy W, Horstman LL, et al. Antiphospholipid antibodies (APLA) in immune thrombocytopenic purpura (ITP) and antiphospholipid syndrome (APS). Am J Hematol, 2006;81(6):391–396
Giordano P, Urbano F, Lassandro G, et al. Role of antithyroid autoimmunity as a predictive biomarker of chronic immune thrombocytopenia. Pediatr Blood Cancer, 2019,66(1):e27452
Carcao MD, Zipursky A, Butchart S, et al. Short-course oral prednisone therapy in children presenting with acute immune thrombocytopenic purpura (ITP). Acta Paediatr Suppl,1998,424:71–74
O’Brien SH, Ritchey AK, Smith KJ. A cost-utility analysis of treatment for acute childhood idiopathic thrombocytopenic purpura(ITP). Pediatr Blood Cancer, 2007,48(2):173–180
Mazzucconi MG, Fazi P, Bernasconi S, et al. Therapy with high-dose dexamethasone (HD-DXM) in previously untreated patients affected by idiopathic thrombocytopenic purpura: a GIMEMA experience. Blood, 2007,109(4):1401–1407
Oved JH, Lee CSY, Bussel JB. Treatment of Children with Persistent and Chronic Idiopathic Thrombocytopenic Purpura: 4 Infusions of Rituximab and Three 4-Day Cycles of Dexamethasone. J Pediatr, 2017,191:225–231
Bussel JB, Lee CS, Seery C, et al. Rituximab and three dexamethasone cycles provide responses similar to splenectomy in women and those with immune thrombocytopenia of less than two years duration. Haematologica, 2014,99(7):1264–1271
Takamatsu H, Feng X, Chuhjo T, et al. Specific antibodies to moesin, a membrane-cytoskeleton linker protein, are frequently detected in patients with acquired aplastic anemia. Blood, 2007,109(6):2514–2520
Hirano N, Butler MO, Von Bergwelt-Baildon MS, et al. Autoantibodies frequently detected in patients with aplastic anemia. Blood, 2003,102(13):4567–4575
Goto M, Kuribayashi K, Takahashi Y, et al. Identification of autoantibodies expressed in acquired aplastic anaemia. Br J Haematol, 2013,160(3):359–362
Ferrara G, Petrillo MG, Giani T, et al. Clinical Use and Molecular Action of Corticosteroids in the Pediatric Age. Int J Mol Sci, 2019,20(2):444
Galon J, Franchimont D, Hiroi N, et al. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J, 2002,16(1): 61–71
Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017,17(4):233–247
Cari L, De Rosa F, Nocentini G, et al. Context-Dependent Effect of Glucocorticoids on the Proliferation, Differentiation, and Apoptosis of Regulatory T Cells: A Review of the Empirical Evidence and Clinical Applications. Int J Mol Sci, 2019,20(5):1142
Szatmari I, Nagy L. Nuclear receptor signalling in dendritic cells connects lipids, the genome and immune function. EMBO J. 2008,27(18):2353–2362
Kojima S, Hibi S, Kosaka Y, et al. Immunosuppressive therapy using antithymocyte globulin, cyclosporine, and danazol with or without human granulocyte colony-stimulating factor in children with acquired aplastic anemia. Blood, 2000,96(6):2049–2054
Narita A, Zhu X, Muramatsu H, et al. Prospective randomized trial comparing two doses of rabbit antithymocyte globulin in patients with severe aplastic anaemia. Br J Haematol, 2019,187(2):227–237
Shallis RM, Ahmad R, Zeidan AM. Aplastic anemia: Etiology, molecular pathogenesis, and emerging concepts. Eur J Haematol, 2018,101(6):711–720
Sabatino JJJr, Pröbstel AK, Zamvil SS. B cells in autoimmune and neurodegenerative central nervous system diseases. Nat Rev Neurosci, 2019,20(12):728–745
Scheinberg P, Nunez O, Weinstein B, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med, 2011,365(5):430–438
Cabannes-Hamy A, Boissel N, Peffault De Latour R, et al. The effect of age in patients with acquired aplastic anaemia treated with immunosuppressive therapy: comparison of Adolescents and Young Adults with children and older adults. Br J Haematol, 2018,183(5): 766–774
Dolberg OJ, Levy Y. Idiopathic aplastic anemia: diagnosis and classification. Autoimmun Rev, 2014, 13(4–5):569–573
Cai B, Said Q, Li X, et al. Healthcare costs and resource utilization in patients with severe aplastic anemia in the US. J Med Econ. 2019,22(10):1055–1062
Hossain MJ, Xie S. Patient features and survival of pediatric aplastic anemia in the USA: a large institution experience. J Public Health (Oxf), 2019,41(2):329–337
Maciejewski JP, Selleri C. Evolution of clonal cytogenetic abnormalities in aplastic anemia. Leuk Lymphoma, 2004,45:433–440
Scheinberg P, Young NS. How I treat aplastic anemia. Blood, 2012,120:1185–1196
Kikuchi A, Yabe H, Kato K, et al. Long-term outcome of childhood aplastic anemia patients who underwent allogeneic hematopoietic SCT from an HLA-matched sibling donor in Japan. Bone Marrow Transplant, 2013,48(5):657–660
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Author Run-ming JIN is a member of the Editorial Board for [Current Medical Science]. The paper was handled by the other editor and has undergone rigorous peer review process. Author Run-ming JIN was not involved in the journal’s review of, or decisions related to, this manuscript.
Additional information
This work was supported by a grant from the National Natural Science Foundation of China (No. 21906061).
Rights and permissions
About this article
Cite this article
Wang, Zj., Chen, Hb., Zhou, F. et al. A New Immunosuppressive Therapy for Very Severe Aplastic Anemia in Children with Autoantibodies. CURR MED SCI 42, 379–386 (2022). https://doi.org/10.1007/s11596-022-2519-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-022-2519-2