Summary
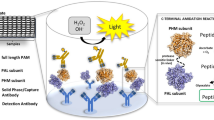
Recombinant batroxobin (S3101) is a thrombin-like serine protease that binds to fibrinogen or is taken up by the reticuloendothelial system. A literature survey showed no adequate method that could determine sufficient concentrations to evaluate pharmacokinetic parameters for phase I clinical studies. Therefore, a sensitive method is urgently needed to support the clinical pharmacokinetic evaluation of S3101. In this study, a sensitive bioanalytical method was developed and validated, using a Quanterix single molecular array (Simoa) assay. Moreover, to thoroughly assess the platform, enzyme-linked immunosorbent assay and electrochemiluminescence assay were also developed, and their performance was compared with that of this novel technology platform. The assay was validated in compliance with the current guidelines. Measurements with the Simoa assay were precise and accurate, presenting a valid assay range from 6.55 to 4000 pg/mL. The intra- and inter-run accuracy and precision were within −19.3% to 15.3% and 5.5% to 17.0%, respectively. S3101 was stable in human serum for 280 days at −20°C and −70°C, for 2 h prior to pre-treatment and 24 h post pre-treatment at room temperature (22°C−28°C), respectively, and after five and two freeze-thaw cycles at −70°C and −20°C, respectively. The Simoa assay also demonstrated sufficient dilution linearity, assay sensitivity, and parallelism for quantifying S3101 in human serum. The Simoa assay is a sensitive and adequate method for evaluating the pharmacokinetic parameters of S3101 in human serum.
Similar content being viewed by others
References
You WK, Choi WS, Koh YS, et al. Functional characterization of recombinant batroxobin, a snake venom thrombin-like enzyme, expressed from Pichia pastoris. FEBS Lett, 2004,571(1–3):67–73
Choi SK, Kim CW, Kim JT, et al. Coagulant effect and tolerability of yeast-produced recombinant batroxobin in healthy adult subjects. Clin Drug Investig, 2018,38(9): 829–835
Ding J, Zhou D, Hu Y, et al. The efficacy and safety of batroxobin in combination with anticoagulation on cerebral venous sinus thrombosis. J Thromb Thrombolysis, 2018,46(3):371–378
Vu TT, Stafford AR, Leslie BA, et al. Batroxobin binds fibrin with higher affinity and promotes clot expansion to a greater extent than thrombin. J Biol Chem, 2013,288(23):16862–16871
Waheed H, Moin SF, Choudhary MI. Snake venom: from deadly toxins to life-saving therapeutics. Curr Med Chem, 2017,24(17):1874–1891
Nagabhushan RM, Shetty AP, Dumpa SR, et al. Effectiveness and safety of batroxobin, tranexamic acid and a combination in reduction of blood loss in lumbar spinal fusion surgery. Spine (Phila Pa 1976), 2018,43(5): E267–E273
Xu G, Liu X, Zhu W, et al. Feasibility of treating hyperfibrinogenemia with intermittently administered batroxobin in patients with ischemic stroke/transient ischemic attack for secondary prevention. Blood Coagul Fibrinolysis, 2007,18(2):193–197
Wang J, Zhu YQ, Liu F, et al. Batroxobin for prevention of restenosis in diabetic patients after infrapopliteal arterial angioplasty: a small randomized pilot trial. Ann Vasc Surg, 2010,24(7):876–884
Oya R, Horii A, Akazawa H, et al. Prognostic predictors of sudden sensorineural hearing loss in defibrinogenation therapy. Acta Otolaryngol, 2016,136(3):271–276
Swamy DF, Barretto ES, Rodrigues JSL. Effectiveness of topical haemocoagulase as a haemostatic agent in children undergoing extraction of primary teeth: a split-mouth, randomised, double-blind, clinical trial. Eur Arch Paediatr Dent, 2019,20(4):311–317
Sugai K, Imamura Y, Mihashi S, et al. Plasma levels and urinary excretion of batroxobin and its defibrinogenating effects in various animal species. J Toxicol Sci, 1986,11(2):135–143
Sugai K, Imamura Y, Mihashi S, et al. Metabolic fate of 125I-labeled batroxobin in rats and dogs. J Toxicol Sci, 1986,11(3):155–167
Zheng ZH, Zhu XX, Gan H, et al. Pharmacodynamics and pharmacokinetics of batroxobin in Beagle dog. Yao Xue Xue Bao, 2013,48(8):1307–1311
Wang R, Fang Y, Pei F, et al. Pharmacokinetics of heamocoagulase agkistrodon injection after a single dose in Chinese healthy volunteers. Chin J Clin Pharmacol (Chinese), 2006,22(6):422–425
NMPA, National Medical Productions Administration, Clinical Pharmacokinetics of Chemical Drugs Technical Guidelines (Chinese)
Chunyk AG, Joyce A, Fischer SK, et al. A multi-site in-depth evaluation of the Quanterix Simoa from a user’s perspective. AAPS J, 2017,20(1):10
Göpfert JC, Reiser A, Carcamo Yañez VA, et al. Development and evaluation of an ultrasensitive free VEGF-A immunoassay for analysis of human aqueous humor. Bioanalysis, 2019,11(9):875–886
ODF, El Aalamat Y, Waelkens E, et al. Multiplex immunoassays in endometriosis: an array of possibilities. Front Biosci (Landmark Ed), 2017,22:479–492
Hendricks R, Baker D, Brumm J, et al. Establishment of neurofilament light chain Simoa assay in cerebrospinal fluid and blood. Bioanalysis, 2019,11(15):1405–1418
Myzithras M, Li H, Bigwarfe T, et al. Development of an ultra-sensitive Simoa assay to enable GDF11 detection: a comparison across bioanalytical platforms. Bioanalysis, 2016,8(6):511–518
US Department of Health and Human Services, US FDA, Center for Drug Evaluation and Research, Biologics Evaluation and Research, Center for Devices and Radiological Health (CDRH). Guidance for Industry, Bioanalytical Method Validation.
European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP). Guideline on Bioanalytical Method Validation, February 2011.
Chinese Pharmacopoeia Commission. Pharmacopoeia of People’s Republic of China (Vol IV). China Medical Science Press, Beijing, China (2015) 360. 9012 Guidelines for method validation (Chinese).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This manuscript has not been published or presented elsewhere in part or in entirety and is not under consideration by another journal. All study participants provided informed consent, and the study design was approved by the appropriate ethics review board. We have read and understood your journal’s policies, and we believe that neither the manuscript nor the study violates any of these. There are no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Wang, Bz., Wang, Mj., Wang, Mr. et al. Development and Validation of a Simoa Assay for Determination of Recombinant Batroxobin in Human Serum. CURR MED SCI 41, 618–625 (2021). https://doi.org/10.1007/s11596-021-2382-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-021-2382-6