Summary
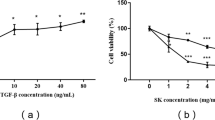
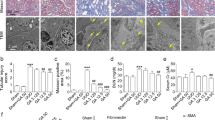
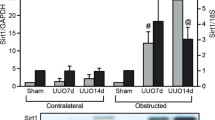
This study aimed to explore the protective effects of the traditional Chinese Medicine formula Shenkang VII recipe (SK-7) on renal fibrosis and the mechanisms. Renal fibrosis was induced by unilateral ureteral obstruction (UUO) in rats. The rats were then divided into 5 groups: control group (Sham operation), UUO model group, UUO model plus low to high doses of SK-7 (0.5, 1.0, or 2.0 g/kg/day, for 14 days) groups. The animals were sacrificed on the 7th or 14th day. Kidney tissues were collected for histopathological examinations (hematoxylin and eosin and Masson’s trichrome staining). Immunohistochemistry was used to detect the expression of collagen type III (Col III), fibronectin (FN), α-smooth muscle actin (α-SMA), TIMP metallopeptidase inhibitor 2 (TIMP2), matrix metallopeptidase 2 (MMP2), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and monocyte chemotactic protein-1 (MCP-1). The TGF-β1/Smad, NF-kB and Sonic hedgehog signaling proteins were detected by Western blotting. Our results showed that SK-7 prevented UUO-induced renal injury and accumulation of collagen fibrils. Renal fibrosis biomarkers Col III, FN, α-SMA and TIMP2 were increased in the rats after UUO and decreased by SK-7, while MMP2 was upregulated after treatment. SK-7 also suppressed the levels of TNF-α, IL-1β and MCP-1 in UUO rats. In addition, SK-7 inhibited activation of the TGF-β/Smad, NF-κB and sonic hedgehog signaling (SHH) pathways. Taken together, these findings suggest that SK-7 may regulate the synthesis and degradation of extracellular matrix, reduce inflammation and suppress the proliferation of fibroblasts, by blocking the TGF-β1/Smad, NF-κB and SHH signaling pathways to exert its anti-renal fibrosis effect in UUO rats.
Similar content being viewed by others
References
Liu JH, He L, Zou ZM, et al. A Novel Inhibitor of Homodimerization Targeting MyD88 Ameliorates Renal Interstitial Fibrosis by Counteracting TGF-βl-Induced EMT in Vivo and in Vitro. Kidney Blood Press Res, 2018,43(5):1677–1687
Humphreys BD.. Mechanisms of Renal Fibrosis. Annu Rev Physiol, 2018,80:309–326
Tang PM, Nikolic-Paterson DJ, Lan HY. Macrophages: versatile players in renal inflammation and fibrosis. Nat Rev Nephrol, 2019,15(3):144–158
Yu Y, Feng XH. TGF-β signaling in cell fate control and cancer. Curr Opin Cell Biol, 2019,61:56–63
Lan HY. Diverse roles of TGF-β/Smads in renal fibrosis and inflammation. Int J Biol Sci, 2011,7(7):1056–1067
Zhou D, Liu Y. Renal fibrosis in 2015: Understanding the mechanisms of kidney fibrosis. Nat Rev Nephrol, 2016,12(2):68–70
Kassiri Z, Defamie V, Hariri M, et al. Simultaneous transforming growth factor beta-tumor necrosis factor activation and cross-talk cause aberrant remodeling response and myocardial fibrosis in Timp3-deficient heart. J Biol Chem, 2009,284(43):29893–29904
Meng XM, Tang PM, Li J, et al.. TGF-β/Smad signaling in renal fibrosis. Front Physiol, 2015,6:82
Ka SM, Huang XR, Lan HY, et al. Smad7 gene therapy ameliorates an autoimmune crescentic glomerulonephritis in mice. J Am Soc Nephrol, 2007,18(6):1777–1788
Liu GX, Li YQ, Huang XR, et al. Disruption of Smad7 promotes ANG II-mediated renal inflammation and fibrosis via Sp1-TGF-β/Smad3-NF. κB-dependent mechanisms in mice. PLoS One, 2013,8(1):e53573
Meng XM.. Inflammatory Mediators and Renal Fibrosis. Adv Exp Med Biol, 2019,1165:381–406
Sutariya B, Jhonsa D, Saraf MN. TGF-β: the connecting link between nephropathy and fibrosis. Immunopharmacol Immunotoxicol, 2016,38(1):39–49
Dennis EA, Smythies LE, Grabski R, et al. Cytomegalovirus promotes intestinal macrophage-mediated mucosal inflammation through induction of Smad7. Mucosal Immunol, 2018,11(6):1694–1704
Huang J, Wan D, Li J, et al. Histone acetyltransferase PCAF regulates inflammatory molecules in the development of renal injury. Epigenetics, 2015,10(1):62–72
Hall ET, Cleverdon ER, Ogden SK. Dispatching Sonic Hedgehog: Molecular Mechanisms Controlling Deployment. Trends Cell Biol, 2019,29(5):385–395
Sasai N, Toriyama M, Kondo T.. Hedgehog Signal and Genetic Disorders. Front Genet, 2019,10:1103
Zhou D, Li Y, Zhou L, et al. Sonic hedgehog is a novel tubule-derived growth factor for interstitial fibroblasts after kidney injury. J Am Soc Nephrol, 2014,25(10):2187–2200
Hong D, Dong Z, Sha H, et al. Sonic hedgehog signaling mediates epithelial-mesenchymal communication and promotes renal fibrosis. J Am Soc Nephrol, 2012,23(5):801–813
Wang Y, Liu N, Su X, et al.. Epigallocatechin-3-gallate attenuates transforming growth factor-β1 induced epithelial-mesenchymal transition via Nrf2 regulation in renal tubular epithelial cells. Biomed Pharmacother, 2015,70:260–267
Zhao YY, Chen H, Tian T, et al. A pharmaco-metabonomic study on chronic kidney disease and therapeutic effect of ergone by UPLC-QTOF/HDMS. PLoS One, 2014,9(12):e115467
Li S, Xiao X, Han L, et al. Renoprotective effect of Zhenwu decoction against renal fibrosis by regulation of oxidative damage and energy metabolism disorder. Sci Rep, 2018,8(1):14627
Jin R, Lin ZJ, Xue CM, et al. An improved association-mining research for exploring Chinese herbal property theory: based on data of the Shennong’s Classic of Materia Medica. J Integr Med, 2013,11(5):352–365
Lin WB, Lin CF, Mei GQ. Analysis of Mei Guo-qiang’s experience in treating intractable diseases with “Situ Decoction”. Shanghai J TCM (Chinese), 2012,46(9):16–17
Martínez-Klimova E, Aparicio-Trejo OE, Tapia E, et al. Unilateral Ureteral Obstruction as a Model to Investigate Fibrosis-Attenuating Treatments. Biomolecules, 2019, 9(4):141
Li A, Zhang X, Shu M, et al.. Arctigenin suppresses renal interstitial fibrosis in a rat model of obstructive nephropathy. Phytomedicine, 2017,30:28–41
Zhao J, Wang L, Cao AL, et al. HuangQi Decoction Ameliorates Renal Fibrosis via TGF-β/Smad Signaling Pathway In Vivo and In Vitro. Cell Physiol Biochem, 2016,38(5):1761–1774
Sampieri CL, Orozco-Ortega RA. Matrix metallopro-teinases and tissue inhibitors of metalloproteinases in chronic kidney disease and acute kidney injury: a systematic review of the literature. Hippokratia, 2018,22(3):99–104
Wang Z, Famulski K, Lee J, et al. TIMP2 and TIMP3 have divergent roles in early renal tubulointerstitial injury. Kidney Int, 2014,85(1):82–93
N El Agha E, Kramann R, Schneider RK, et al. Mesenchymal Stem Cells in Fibrotic Disease. Cell Stem Cell, 2017,21(2):166–177
Li JH, Zhu HJ, Huang XR, et al. Smad7 inhibits fibrotic effect of TGF-Beta on renal tubular epithelial cells by blocking Smad2 activation [published correction appears in J Am Soc Nephrol. 2003 Sep;14(9):2417]. J Am Soc Nephrol, 2002,13(6):1464–1472
Zhang ZH, Li MH, Liu D, et al.. Rhubarb Protect Against Tubulointerstitial Fibrosis by Inhibiting TGF-β/Smad Pathway and Improving Abnormal Metabolome in Chronic Kidney Disease. Front Pharmacol, 2018,9:1029
Hu HH, Chen DQ, Wang YN, et al.. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem Biol Interact, 2018,292:76–83
House CD, Grajales V, Ozaki M, et al. IKKε cooperates with either MEK or non-canonical NF-kB driving growth of triple-negative breast cancer cells in different contexts. BMC Cancer, 2018,18(1):595
Shih RH, Wang CY, Yang CM. NF-kappaB Signaling Pathways in Neurological Inflammation: A Mini Review. Front Mol Neurosci, 2015,8:77
Tornatore L, Thotakura AK, Bennett J, et al. The nuclear factor kappa B signaling pathway: integrating metabolism with inflammation. Trends Cell Biol, 2012,22(11):557–566
Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res, 2011,21(1):103–115
Sun SC. The non-canonical NF-κB pathway in immunity and inflammation. Nat Rev Immunol, 2017,17(9):545–558
Voges D, Zwickl P, Baumeister W.. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu Rev Biochem, 1999,68:1015–1068
Schütz E, Bochenek ML, Riehl DR, et al. Absence of transforming growth factor beta 1 in murine platelets reduces neointima formation without affecting arterial thrombosis. Thromb Haemost, 2017,117(9):1782–1797
Du J, Paz K, Flynn R, et al. Pirfenidone ameliorates murine chronic GVHD through inhibition of macrophage infiltration and TGF-β production. Blood, 2017,129(18):2570–2580
Chung AC, Huang XR, Zhou L, et al. Disruption of the Smad7 gene promotes renal fibrosis and inflammation in unilateral ureteral obstruction (UUO) in mice. Nephrol Dial Transplant, 2009,24(5):1443–1454
Lin N, Ji Z, Huang C. Smad7 alleviates glomerular mesangial cell proliferation via the ROS-NF-κB pathway. Exp Cell Res, 2017,361(2):210–216
Zhou D, Tan RJ, Liu Y. Sonic hedgehog signaling in kidney fibrosis: a master communicator. Sci China Life Sci, 2016,59(9):920–929
Liu X, Sun N, Mo N, et al. Quercetin inhibits kidney fibrosis and the epithelial to mesenchymal transition of the renal tubular system involving suppression of the Sonic Hedgehog signaling pathway. Food Funct, 2019,10(6):3782–3797
Lu H, Chen B, Hong W, et al. Transforming growth factor-β1 stimulates hedgehog signaling to promote epithelial-mesenchymal transition after kidney injury. FEBS J, 2016,283(20):3771–3790
Cruz-Solbes AS, Youker K.. Epithelial to Mesenchymal Transition (EMT) and Endothelial to Mesenchymal Transition (EndMT): Role and Implications in Kidney Fibrosis. Results Probl Cell Differ, 2017,60:345–372
Devocelle A, Lecru L, François H, et al. Inhibition of TGF-β1 Signaling by IL-15: A Novel Role for IL-15 in the Control of Renal Epithelial-Mesenchymal Transition: IL-15 Counteracts TGF-β1-Induced EMT in Renal Fibrosis. Int J Cell Biol, 2019:9151394
Kramann R. Hedgehog Gli signalling in kidney fibrosis. Nephrol Dial Transplant, 2016,31(12):1989–1995
Ji GQ, Chen RQ, Wang L. Anti-inflammatory activity of atractylenolide III through inhibition of nuclear factor-κB and mitogen-activated protein kinase pathways in mouse macrophages. Immunopharmacol Immunotoxicol, 2016,38(2):98–102
Yang J, Zeng Z, Wu T, et al. Emodin attenuates high glucose-induced TGF-β1 and fibronectin expression in mesangial cells through inhibition of NF-κB pathway. Exp Cell Res, 2013,319(20):3182–3189
Chen F, Zhu X, Sun Z, et al.. Astilbin Inhibits High Glucose-Induced Inflammation and Extracellular Matrix Accumulation by Suppressing the TLR4/MyD88/NF-κB Pathway in Rat Glomerular Mesangial Cells. Front Pharmacol, 2018,9:1187
Chen JK, Guo MK, Bai XH, et al. Astragaloside IV ameliorates intermittent hypoxia-induced inflammatory dysfunction by suppressing MAPK/NF-κB signalling pathways in Beas-2B cells. Sleep Breath, 2020,24(3):1237–1245
Gao Y, Hou R, Liu F, et al. Obacunone causes sustained expression of MKP-1 thus inactivating p38 MAPK to suppress pro-inflammatory mediators through intracellular MIF. J Cell Biochem, 2018,119(1):837–849
Tsang SW, Zhang H, Lin C, et al. Rhein, a natural anthraquinone derivative, attenuates the activation of pancreatic stellate cells and ameliorates pancreatic fibrosis in mice with experimental chronic pancreatitis. PLoS One, 2013,8(12):e82201
Ma L, Li H, Zhang S, et al. Emodin ameliorates renal fibrosis in rats via TGF-β1/Smad signaling pathway and function study of Smurf 2. Int Urol Nephrol, 2018,50(2):373–382
Qu W, Wang Y, Wu Q, et al.. Emodin Impairs Radioresistance of Human Osteosarcoma Cells by Suppressing Sonic Hedgehog Signaling. Med Sci Monit, 2017,23:5767–5773
Chen L, Lan Z, Zhou Y, et al. Astilbin attenuates hyperuricemia and ameliorates nephropathy in fructose-induced hyperuricemic rats. Planta Med, 2011,77(16):1769–1773
Chen F, Zhu X, Sun Z, et al.. Astilbin Inhibits High Glucose-Induced Inflammation and Extracellular Matrix Accumulation by Suppressing the TLR4/MyD88/NF-κB Pathway in Rat Glomerular Mesangial Cells. Front Pharmacol, 2018,9:1187
Guo LH, Cao Y, Zhuang RT, et al. Astragaloside IV promotes the proliferation and migration of osteoblast-like cells through the hedgehog signaling pathway. Int J Mol Med, 2019,43(2):830–838
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by Academic Experience Inheritance of the Sixth National Group of Old Chinese Medicine Experts of the State Administration of Traditional Chinese Medicine (No. 2017 [29]), and the key projects of Hubei Provincial Department of Health (No. JX6A09).
Conflict of Interest Statement
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhou, Ss., Ai, Zz., Li, Wn. et al. Shenkang VII Recipe Attenuates Unilateral Ureteral Obstruction-induced Renal Fibrosis via TGF-β/Smad, NF-κB and SHH Signaling Pathway. CURR MED SCI 40, 917–930 (2020). https://doi.org/10.1007/s11596-020-2255-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-020-2255-4