Summary
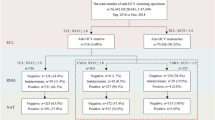
The study investigated the distribution of Epstein-Barr virus (EBV) EA-IgA, VCA-IgA, and EBVNA-IgG antibodies in a local population of Wuhan, China. Chemiluminescence immunoassay (CLIA) was used to detect EBV EA-IgA, VCA-IgA, and EBVNA-IgG antibodies in 972 subjects undergoing physical examination in Wuhan, and the results were analyzed. The detection rate of EBV was positively correlated with age. In the 972 cases, there was significant difference between different genders in the positive rate of VCA-IgA and EBVNA-IgG. Moreover, the positive rate of VCA-IgA and EBVNA-IgG was higher in men ≥ 60 years old than in those < 60 but no significant differences were found in three antibodies among various age groups. Our results suggested that the EBV infection should be intensively monitored in elderly people in Wuhan.
Similar content being viewed by others
References
Thompson MP, Kurzrock R. Epstein-Barr virus and cancer. Clin Cancer Res, 2004,10(3):803–821
Wu ZR, Li G, Wu L, et al. Cripto-1 overexpression is involved in the tumorigenesis of nasopharyngeal carcinoma. BMC Cancer, 2009,315(9):1–14
Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet, 2005,365(9476):2041–2054
Cheng WM, Chan KH, Chen HL, et al. Assessing the risk of nasopharyngeal carcinoma on the basis of EBV antibody spectrum. Int J Cancer, 2002,97(4):489–492
Wu WH, Chen GX, Wu ZB, et al. Serological detection of EB virus in early detection and screening of nasopharyngeal carcinoma. Cancer, 2006,25(2):250–256
Peng ZX, Huang ZY, Tang YL, et al. Application of EB virus antibody detection in nasopharyngeal carcinoma class II prevention. Guangxi Med (Chinese), 1999,21(4):611–614
Chen P, Xiao ZY. Detection of antibodies to EB virus VCA-IgA in 1367 employees of a university in Fuzhou. J Fujian Med Univ (Chinese), 2006,40(1):86–92
Deng H, Ceng Y, Zheng YM, et al. Serological survey of 413 164 nasopharyngeal carcinoma in natural population. Chin Oncol, 2003,13(2):109–111
Deng H, Jing Y, Zheng YM, et al. Serological survey of nasopharyngeal carcinoma in 413 164 natural population. Chin Oncol, 2007,23(7):492–493
Li Y. Investigation of EB virus VCA/IgA in 16 873 people undergoing physical examination. Wenxuan Med (Chinese), 2005,24(6):894–895
Shebl FM, Bhatia K, Engels EA. Salivary gland and nasopharyngeal cancers in individuals with acquired immunodeficiency syndrome in United States. Int J Cancer, 2010,126:2503–2508
Liang HT, Ye Y, Chen L, et al. Investigation and analysis of EB virus infection among Guangzhou health examination personnel. Cli Med Engineering, 2010,17(6):131–132
Okan M. Overview and problematic standpoints of severe chronic Epstein-Barr virus infection syndrome. Oncol Hematol, 2002,44(4):273–282
Han TT, Xu LP, Liu DH. Analysis of EB virus infection after allogeneic hematopoietic stem cell transplantation. Chin J Hematol (Chinese), 2013,8(6):651–654
Draborg AH, Duus K, Houen G. Epstein-Bar virus in systemic autoimmune diseases. Clin Dev Immunol, 2013,8(1):1–9
Wang PP, Ji MF. Research progress in serological screening of nasopharyngeal carcinoma. Tumor Res Bedside, 2013,8(5):572–573
Acknowledgments
The authors would like to thank Mei-hong ZENG and Yan SHEN of the Physical Examination Center, Union Hospital for their help with participant recruitment, and Liqin ZHU of the Physical Examination Center, Union Hospital for her assistance in laboratory management.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Conflict of Interest Statement
The authors declare that there is no conflict of interest with any financial organization or corporation or individual that can inappropriately influence this work.
This project was supported by grants from the Natural Science Foundation of Hubei Province (No. 2018CFB749 and No. 2018ADC073), the Scientific Research Fund of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. 02.03.2017-292 and No. 02.03.2017-327) and General Project of Hubei Health Committee (No. 02.07.19030018).
Rights and permissions
About this article
Cite this article
Tang, Zg., Li, Hh., Li, Yc. et al. Epstein-Barr Virus EA-IgA, VCA-IgA, and EBVNA-IgG Antibodies in a Population of Wuhan, China. CURR MED SCI 40, 168–171 (2020). https://doi.org/10.1007/s11596-020-2160-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-020-2160-x