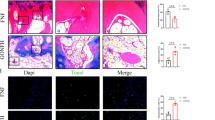
Abstract
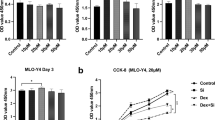
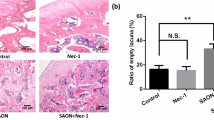
Nowadays, the cumulative intake of glucocorticoids has become the most common pathogenic factor for non-traumatic osteonecrosis of the femoral head (ONFH). Apoptosis of osteoblasts is considered as the main reason of ONFH at the molecular level. Glycogen synthase kinase 3β (GSK3β) is an important regulator of cellular differentiation and apoptosis pathway, which can modulate the balance between osteoblasts and osteoclasts. Several studies have reported about its function in osteoporosis, but little is known about it in osteonecrosis. In our study, lipopolysaccharide and methylprednisolone were utilized to establish a rat ONFH model. The phosphorylation of GSK3β Ser-9 was decreased in the model. Western blotting examination of β-catenin, Bcl-2, Bax and caspase-3 revealed that the osteoblasts were apoptotic. In dexamethasone (Dex)-incubated primary osteoblasts, the expression profile of GSK3β phosphorylation and apoptotic factors were consistent with those in the rat ONFH model. To further investigate the regulation of osteonecrosis caused by GSK3β, the expression and function of GSK3β were inhibited in Dex-incubated primary osteoblasts. The knockdown of GSK3β by siRNA decreased the expression of Bax and cleaved caspase-3, but increased Bcl-2 and β-catenin. On the other hand, selective inhibition of GSK3β function by LiCl counteracted the activation of caspase-3 induced by Dex. Our work is the first study about the GSK3β phosphorylation in ONFH, and provides evidence for further therapeutic methods.
Similar content being viewed by others
References
Gessi S, Merighi S, Borea PA. Glucocorticoid’s pharmacology: past, present and future. Curr Pharm Des, 2010,16(32):3540–3553
Yang Z, Liu H, Li D, et al. The efficacy of statins in preventing glucocorticoid–related osteonecrosis in animal models: A meta–analysis. Bone Joint Res, 2016,5(9):393–402
Zheng H, Yang E, Peng H, et al. Gastrodin prevents steroid–induced osteonecrosis of the femoral head in rats by anti–apoptosis. Chin Med J, 2014,127(22):3926–3931
Weinstein RS. Glucocorticoid–induced osteoporosis and osteonecrosis. Endocrinol Metab Clin North Am, 2012,41(3):595–611
Zalavras C, Shah S, Birnbaum MJ, et al. Role of apoptosis in glucocorticoid–induced osteoporosis and osteonecrosis. Crit Rev Eukaryot Gene, 2003,13(2–4):221–235
Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase–3. Trends Biochem Sci, 2004,29(2):95–102
Yun SI, Yoon HY, Jeong SY, et al. Glucocorticoid induces apoptosis of osteoblast cells through the activation of glycogen synthase kinase 3beta. J Bone Miner Metab, 2009,27(2):140–148
Guo B, Zhang W, Xu S, et al. GSK–3beta mediates dexamethasone–induced pancreatic beta cell apoptosis. Life Sci, 2016,144:1–7
Spokoini R, Kfir–Erenfeld S, Yefenof E, et al. Glycogen synthase kinase–3 plays a central role in mediating glucocorticoid–induced apoptosis. Mol Endocrinol, 2010,24(6):1136–1150
Hughes K, Nikolakaki E, Plyte SE, et al. Modulation of the glycogen synthase kinase–3 family by tyrosine phosphorylation. EMBO J, 1993,12(2):803–808
Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. P Natl Acad Sci USA, 1996,93(16):8455–8459
Eldar–Finkelman H. Glycogen synthase kinase 3: an emerging therapeutic target. Trends Mol Med, 2002,8(3):126–132
Martinez A, Castro A, Dorronsoro I, et al. Glycogen synthase kinase 3 (GSK–3) inhibitors as new promising drugs for diabetes, neurodegeneration, cancer, and inflammation. Med Res Rev, 2002,22(4):373–384
Sharma M, Chuang WW, Sun Z. Phosphatidylinositol 3–kinase/Akt stimulates androgen pathway through GSK3beta inhibition and nuclear beta–catenin accumulation. J Biol Chem, 2002,277(34):30 935–30 941
Song L, De Sarno P, Jope RS. Central role of glycogen synthase kinase–3beta in endoplasmic reticulum stress–induced caspase–3 activation. J Biol Chem, 2002,277(47):44 701–44 708
Jin ZH, Kurosu T, Yamaguchi M, et al. Hematopoietic cytokines enhance Chk1–dependent G2/M checkpoint activation by etoposide through the Akt/GSK3 pathway to inhibit apoptosis. Oncogene, 2005,24(12):1973–1981
Zhang C, Zou YL, Ma J, et al. Apoptosis associated with Wnt/beta–catenin pathway leads to steroid–induced avascular necrosis of femoral head. BMC Musculoskel Dis, 2015,16:132
Smith E, Coetzee GA, Frenkel B. Glucocorticoids inhibit cell cycle progression in differentiating osteoblasts via glycogen synthase kinase–3beta. J Biol Chem, 2002,277(20):18 191–18 197
Okazaki S, Nishitani Y, Nagoya S, et al. Femoral head osteonecrosis can be caused by disruption of the systemic immune response via the toll–like receptor 4 signalling pathway. Rheumatology, 2009,48(3):227–232
Li H, Qian W, Weng X, et al. Glucocorticoid receptor and sequential P53 activation by dexamethasone mediates apoptosis and cell cycle arrest of osteoblastic MC3T3–E1 cells. PLoS one, 2012,7(6):e37 030
Li J, He C, Tong W, et al. Tanshinone IIA blocks dexamethasone–induced apoptosis in osteoblasts through inhibiting Nox4–derived ROS production. Int J Clin Exp Patho, 2015,8(10):13 695–13 706
Sato AY, Tu X, McAndrews KA, et al. Prevention of glucocorticoid induced–apoptosis of osteoblasts and osteocytes by protecting against endoplasmic reticulum (ER) stress in vitro and in vivo in female mice. Bone, 2015,73:60–68
Beurel E, Jope RS. The paradoxical pro–and antiapoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol, 2006,79(4):173–189
Akhtar RS, Ness JM, Roth KA. Bcl–2 family regulation of neuronal development and neurodegeneration. BBAMol Cell Res, 2004,1644(2–3):189–203
Bijur GN, Jope RS. Glycogen synthase kinase–3 beta is highly activated in nuclei and mitochondria. Neuroreport, 2003,14(18):2415–2419
Watcharasit P, Bijur GN, Song L, et al. Glycogen synthase kinase–3beta (GSK3beta) binds to and promotes the actions of p53. J Biol Chem, 2003,278(49):48 872–48 879
Guidotti S, Minguzzi M, Platano D, et al. Lithium Chloride Dependent Glycogen Synthase Kinase 3 Inactivation Links Oxidative DNA Damage, Hypertrophy and Senescence in Human Articular Chondrocytes and Reproduces Chondrocyte Phenotype of Obese Osteoarthritis Patients. PLoS One, 2015,10(11):e0143 865
Li H, Huang K, Liu X, et al. Lithium chloride suppresses colorectal cancer cell survival and proliferation through ROS/GSK–3beta/NF–kappaB signaling pathway. Oxid Med Cell Longev, 2014,2014:241 864
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by the National Natural Science Foundation of China (No. 81672154).
Rights and permissions
About this article
Cite this article
Deng, S., Nie, Zg., Peng, Pj. et al. Decrease of GSK3β Ser-9 Phosphorylation Induced Osteoblast Apoptosis in Rat Osteoarthritis Model. CURR MED SCI 39, 75–80 (2019). https://doi.org/10.1007/s11596-019-2002-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-019-2002-x