Summary
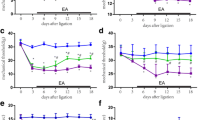
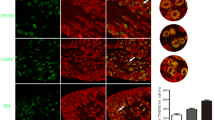
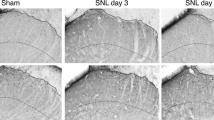
This study investigated the effects of different frequencies of repetitive transcranial magnetic stimulation (rTMS) on chronic neuropathic pain in rats. The behavior of rats with experimental chronic neuropathic pain was observed, and the expression of neuronal nitric oxide synthase (nNOS) in the ipsilateral dorsal root ganglions (DRGs) and the activation and proliferation of astrocytes in the ipsilateral spinal dorsal horn were detected. Thirty-two male Sprague-Dawley rats were randomly divided into four groups: sham-operated group, sham-rTMS group, 1 Hz group and 20 Hz group (8 rats in each group). Chronic constriction nerve injury induced by sciatic nerve ligation was made to establish the models of the chronic neuropathic pain in rats except those in the sham-operated group. Then we applied different frequencies of rTMS to the primary motor cortex (Ml) contralateral to the pain side once daily for 10 consecutive days. Pain behavior scores were observed before and after treatment. Western blot analysis was used to detect the expression of nNOS in ipsilateral L4-6 DRGs. Double immunofluorescent labeling for glial fibrillary acidic protein (GFAP) and 5-bromo-2- deoxyuridine (BrdU) was employed to observe the activation and proliferation of astrocytes in the ipsilateral L4-6 spinal dorsal horn. After rTMS treatment, the spontaneous pain behavior scores were significantly lower in the 20 Hz group than those in the sham-rTMS group (P<0.05). Moreover, the brush-evoked pain behavior scores were significantly lower in the 20 Hz group than those in the sham-rTMS and 1 Hz group (P<0.05), suggesting that the spontaneous pain and brush-evoked pain in the 20 Hz group were significantly alleviated. Western blot analysis revealed that the expression of nNOS in ipsilateral L4-6 DRGs was significantly decreased in the 20 Hz group as compared with the sham-rTMS group and the 1 Hz group (P<0.01) after rTMS treatment. Double immunofluorescence suggested that the expression of GFAP and the co-localization with BrdU in astrocytes were less in the sham-operated group than those in the sham-rTMS group and the 1 Hz group in L4-6 spinal dorsal horn ipsilateral to the neuropathic pain. After rTMS treatment, the expression of GFAP and the co-localization with BrdU decreased in the 20 Hz group as compared with the sham-rTMS group and the 1 Hz group (P<0.05). In addition, the alleviation degree of spontaneous pain and brush-evoked pain in the 20 Hz group was negatively correlated with the expression of nNOS in ipsilateral DRGs and the number of GFAP/BrdU co-labelled astrocytes in L4-6 spinal dorsal horn ipsilateral to the neuropathic pain (P<0.05). It was suggested that high-frequency rTMS may relieve neuropathic pain through down-regulating the overexpression of nNOS in ipsilateral DRGs and inhibiting the activity and proliferation of astrocytes in L4-6 spinal dorsal horn ipsilateral to the neuropathic pain.
Similar content being viewed by others
References
Plow EB, Pascual-Leone A, Machado A. Brain stimulation in the treatment of chronic neuropathic and non-cancerous pain. J Pain, 2012,13(5):411–424
Lefaucheur JP. Use of repetitive transcranial magnetic stimulation in pain relief. Expert Rev Neurother, 2008,8(5):799–808
Canavero S, Bonicalzi V, Dotta M, et al. Low-rate repetitive TMS allays central pain. Neurol Res, 2003,25(2): 151–152
Khedr EM, Kotb H, Kamel NF, et al. Longlasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain. J Neurol Neurosurg Psychiatry, 2005,76(6):833–838
Cizkova D, Lukacova N, Marsala M, et al. Neuropathic pain is associated with alterations of nitric oxide synthase immunoreactivity and catalytic activity in dorsal root ganglia and spinal dorsal hom. Brain Res Bull, 2002,58(2):161–171
Kim KH, Kim JI, Han JA, et al. Upregulation of neuronal nitric oxide synthase in the periphery promotes pain hypersensitivity after peripheral nerve injury. Neuroscience, 2011,190:367–378
Zhang X, Verge V, Wiesenfeld-Hallin Z, et al. Nitric oxide synthase-like immunoreactivity in lumbar dorsal root ganglia and spinal cord of rat and monkey and effect of peripheral axotomy. J Comp Neurol, 1993,335(4):563–575
Choi Y, Raja SN, Moore LC, et al. Neuropathic pain in rats is associated with altered nitric oxide synthase activity in neural tissue. J Neurol Sci, 1996,138(1-2): 14–20
Zhuo M, Wu G, Wu LJ. Neuronal and microglial mechanisms of neuropathic pain. Mol Brain, 2011,4:31
Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nat Rev Drug Discov, 2003,2(12):973–985
Zhang J, De Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem, 2006,97(3):772–783
Garrison CJ, Dougherty PM, Kajander KC, et al. Staining of glial fibrillary acidic protein (GFAP) in lumbar spinal cord increases following a sciatic nerve constriction injury. Brain Res, 1991,565(1): 1–7
Andre-Obadia N, Peyron R, Mertens P, et al. Transcranial magnetic stimulation for pain control. Double-blind study of different frequencies against placebo, and correlation with motor cortex stimulation efficacy. ClinNeurophysiol, 2006,117(7):1536–1544
O'Rielly DD, Loomis CW. Increased expression of cyclooxygenase and nitric oxide isoforms, and exaggerated sensitivity to prostaglandin E2, in the rat lumbar spinal cord 3 days after L5-L6 spinal nerve ligation. Anesthesiology, 2006,104(2):328–337
Kozel FA, George MS. Meta-analysis of left prefrontal repetitive transcranial magnetic stimulation (rTMS) to treat depression. J Psychiatr Pract, 2002,8(5):270–275
Wassermann EM, Lisanby SH. Therapeutic application of repetitive transcranial magnetic stimulation: a review. ClinNeurophysiol, 2001,112(8):1367–1377
Gao W, Yu LG, Liu YL, etal. Effects of high frequency repetitive transcranial magnetic stimulation on kcc2 expression in rats with spasticity following spinal cord injury. J Huazhong Univ Sci Technolog Med Sci, 2017,37(5):777–781
Leo RJ, Latif T. Repetitive transcranial magnetic stimulation (rTMS) in experimentally induced and chronic neuropathic pain: a review. J Pain, 2007,8(6):453–459
Lefaucheur JP. New insights into the therapeutic potential of non-invasive transcranial cortical stimulation in chronic neuropathic pain. Pain, 2006,122(1-2): 11–13
Lefaucheur JP, Hatem S, Nineb A, et al. Somatotopic organization of the analgesic effects of motor cortex rTMS in neuropathic pain. Neurology, 2006,67(11): 1998–2004
Hirayama A. Reduction of intractable deafferentation pain by navigation-guided repetitive transcranial magnetic stimulation of the primary motor cortex. Pain, 2006,122(1-2):22–27
Rollnik JD. Repetitive transcranial magnetic stimulation for the treatment of chronic pain~a pilot study. Eur Neurol, 2002,48(1):6–10
Lefaucheur JP, Drouot X, Nguyen JP. Interventional neurophysiology for pain control: duration of pain relief following repetitive transcranial magnetic stimulation of the motor cortex. Neurophysiol Clin, 2001,31(4):247–252
Lefaucheur JP. Neuropathic pain controlled for more than a year by monthly sessions of repetitive transcranial magnetic stimulation of the motor cortex. Neurophysiol Clin, 2004,34(2):91–95
Lefaucheur JP. Neurogenic pain relief by repetitive transcranial magnetic cortical stimulation depends on the origin and the site of pain. J Neurol Neurosurg Psychiatry, 2004,75(4):612–616
Bestmann S. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci, 2004,19(7): 1950–1962
Pleger B. Repetitive transcranial magnetic stimulation of the motor cortex attenuates pain perception in complex regional pain syndrome type I. Neurosci Lett, 2004,356(2):87–90
Garcia-Larrea L, Peyron R, Mertens P, et al. Electrical stimulation of motor cortex for pain control: a combined PET-scan and electrophysiological study. Pain, 1999,83(2):259–273
Lefaucheur JP, Drouot X, Keravel Y, et al. Pain relief induced by repetitive transcranial magnetic stimulation of precentral cortex. Neuroreport, 2001,12(13):2963–2965
Qian Y, Chao DS, Santillano DR, et al. cGMPdependent protein kinase in dorsal root ganglion: relationship with nitric oxide synthase and nociceptive neurons. J Neurosci, 1996,16(10):3130–3138
Schmidt H, Werner M, Heppenstall PA, et al. cGMPmediated signaling via cGKIalpha is required for the guidance and connectivity of sensory axons. J Cell Biol, 2002,159(3):489–498
Garthwaite J, Charles SL, Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature, 1988,336(6197):385–388
Tegeder I, Del TD, Schmidtko A, et al. Reduced inflammatory hyperalgesia with preservation of acute thermal nociception in mice lacking cGMPdependent protein kinase I. Proc Natl Acad Sci USA, 2004,101(9):3253–3257
MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol, 1997,15:323–350
Aimi Y, Fujimura M, Vincent SR, et al. Localization of NADPH-diaphorase-containing neurons in sensory ganglia of the rat. J Comp Neurol, 1991,306(3):382–392
Kim SJ, Song SK, Kim J. Inhibitory effect of nitric oxide on voltage-dependent calcium currents in rat dorsal root ganglion cells. Biochem Biophys Res Commun, 2000,271(2):509–514
Guan Y, Yaster M, Raja SN, et al. Genetic knockout and pharmacologic inhibition of neuronal nitric oxide synthase attenuate nerve injury-induced mechanical hypersensitivity in mice. Mol Pain, 2007,3:29
Shortland PJ. ATF3 expression in L4 dorsal root ganglion neurons after L5 spinal nerve transection. Eur J Neurosci, 2006,23(2):365–373
Luo ZD. Neuronal nitric oxide synthase mRNA upregulation in rat sensory neurons after spinal nerve ligation: lack of a role in allodynia development. J Neurosci, 1999,19(21):9201–9208
Naik AK, Tandan SK, Kumar D, et al. Nitric oxide and its modulators in chronic constriction injuryinduced neuropathic pain in rats. Eur J Pharmacol, 2006,530(l-2):59–69
Hao JX, Xu XJ. Treatment of a chronic allodynialike response in spinally injured rats: effects of systemically administered nitric oxide synthase inhibitors. Pain, 1996,66(2-3):313–319
Echeverry S, Shi XQ, Zhang J. Characterization of cell proliferation in rat spinal cord following peripheral nerve injury and the relationship with neuropathic pain. Pain, 2008,135(l-2):37–47
Nesic O, Lee J, Johnson KM, et al. Transcriptional profiling of spinal cord injury-induced central neuropathic pain. J Neurochem, 2005,95(4):998–1014
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, L., Wang, Sh., Hu, Y. et al. Effects of Repetitive Transcranial Magnetic Stimulation on Astrocytes Proliferation and nNOS Expression in Neuropathic Pain Rats. CURR MED SCI 38, 482–490 (2018). https://doi.org/10.1007/s11596-018-1904-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-018-1904-3