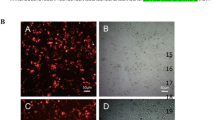
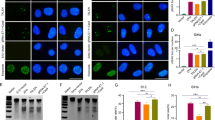
Summary
The objectives of this study were to investigate the effects of the CRISPR/Cas9 system mediated by the HPV pseudotype virus on SiHa cytobiology behavior by cutting the HPV16 E6 gene selectively and to explore the role of this system in the treatment of cervical cancer. After designing specific gRNA sequences targeting HPV16 E6, generating hCas9-EGFP and E6-gRNA-RFP plasmids, and preparing the pseudovirus of HPV16 carrying E6-gRNA and Cas9 plasmids, we determined the titer of the pseudotype virus using the TCID50 method. We obtained the pseudotype virus of HPV16 carrying E6-gRNA and Cas9 plasmids to transfect cervical cancer SiHa cells. Experimental subjects were divided into control group, empty virus group, E6-gRNA transfected group, Cas9 transfected group and Cas9+E6-gRNA transfected group. The molecular size of the cutting sequence was detected using the T7E1 enzyme digestion method and agarose gel electrophoresis, and the cleavage function of CRISPR/Cas9 on the E6 gene was determined at the same time. RT-PCR and Western blotting were performed to detect the mRNA and protein expression levels of E6 in all the groups; the Transwell cell migration assay was performed to detect the cell migration ability and metastasis in all groups. Heterotopic transplantation tumors were incorporated into mice and were used to investigate the effects of the CRISPR/Cas9 system mediated by the HPV pseudovirus on the tumorigenic ability of SiHa cells by selectively cutting HPV16 E6. The HPV16 pseudotype virus carrying E6-gRNA and Cas9 plasmids could successfully infect SiHa cells, and there were two cutting zones in the Cas9+E6-gRNA transfected group. However, the empty virus group, E6-gRNA transfected group and Cas9 transfected group had no corresponding zone. Compared with those in the control group, the empty virus group, E6-gRNA transfected group and Cas9 transfected group, the mRNA and protein expression levels of E6 in SiHa cells were downregulated in the Cas9+E6-gRNA transfected group (P<0.01). In addition, the proliferation and migration abilities of SiHa cells were significantly inhibited (P<0.01). There were no significant differences among the other groups. In contrast to the control group, the HPV pseudotype virus carrying E6-gRNA and Cas9 plasmids could significantly delay the growth of tumor cells of the ectopic tumor transplantation model (P<0.01). The CRISPR/Cas9 system mediated by the HPV pseudotype virus to knockout E6 gene expression exhibited a clear inhibitory effect on the biological function of SiHa cells, which indicated that knocking out the E6 gene using the CRISPR/Cas9 system mediated by the HPV pseudotype virus had a potential effect of eliminating HPV infection and inhibiting the growth of HPV-related tumors. Taken together, these findings provide insight into a new treatment strategy for the prevention and treatment of hr-HPV infected disease, particularly in HPV-related tumors.
Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin, 2015,65(2):87–108
Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond), 2006,110(5):525–541
Sasano H, Yazdani S,Kasajima A. WHO classification of pancreatic neuroendocrine tumor: overview. Nihon Rinsho, 2015,73(Suppl 3):321–326
Li K, Jin X, Fang Y, et al. Correlation between physical status of human papilloma virus and cervical carcinogenesis. J Huazhong Univ Sci Technolog Med Sci, 2012,32(1):97–102
Hopman AH, Theelen W, Hommelberg PP, et al. Genomic integration of oncogenic HPV and gain of the human telomerase gene TERC at 3q26 are strongly associated events in the progression of uterine cervical dysplasia to invasive cancer. J Pathol, 2006,210(4):412–419
Melsheimer P, Vinokurova S, Wentzensen N, et al. DNA aneuploidy and integration of human papillomavirus type 16 e6/e7 oncogenes in intraepithelial neoplasia and invasive squamous cell carcinoma of the cervix uteri. Clin Cancer Res, 2004,10(9):3059–3063
Murphy N, Ring M, Killalea AG, et al. p16INK4A as a marker for cervical dyskaryosis: CIN and cGIN in cervical biopsies and ThinPrep smears. J Clin Pathol, 2003,56(1):56–63
Klaes R, Benner A, Friedrich T, et al. p16INK4a immunohistochemistry improves interobserver agreement in the diagnosis of cervical intraepithelial neoplasia. Am J Surg Pathol, 2002,26(11):1389–1399
Lin SR, Yang HC, Kuo YT, et al. The CRISPR/Cas9 System Facilitates Clearance of the Intrahepatic HBV Templates In Vivo. Mol Ther Nucleic Acids, 2014,3(8):e186
Yuen KS, Chan CP, Wong NH, et al. CRISPR/Cas9-mediated genome editing of Epstein-Barr virus in human cells. J Gen Virol, 2015,96 (Pt 3):626–636
Wang J, Quake SR. RNA-guided endonuclease provides a therapeutic strategy to cure latent herpesviridae infection. Proc Natl Acad Sci USA, 2014,111(36):13157–13162
Kennedy EM, Kornepati AV, Goldstein M, et al. Inactivation of the human papillomavirus E6 or E7 gene in cervical carcinoma cells by using a bacterial CRISPR/Cas RNA-guided endonuclease. J Virol, 2014,88(20):11965–11972
Gao F, Shen XZ, Jiang F, et al. DNA-guided genome editing using the Natronobacterium gregoryi Argonaute. Nat Biotechnol, 2016,34(7):768–773
Buck CB, Pastrana DV, Lowy DR, et al. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol Med, 2005,119:445–462
Stemmer M, Thumberger T, Del Sol Keyer M, et al. CCTop: An Intuitive, Flexible and Reliable CRISPR/Cas9 Target Prediction Tool. PLoS One, 2015,10(4):e0124633
D’Costa ZJ, Jolly C, Androphy EJ, et al. Transcriptional repression of E-cadherin by human papillomavirus type 16 E6. PLoS One, 2012,7(11):e48954
Ebina H, Misawa N, Kanemura Y, et al. Harnessing the CRISPR/Cas9 system to disrupt latent HIV-1 provirus. Sci Rep, 2013,3:2510
Gaj T, Gersbach CA,Barbas CF 3rd. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol, 2013,31(7):397–405.
Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell, 2014,157(6):1262–1278
Platt RJ, Chen S, Zhou Y, et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell, 2014,159(2):440–455
Xue W, Chen S, Yin H, et al. CRISPR-mediated direct mutation of cancer genes in the mouse liver. Nature, 2014,514(7522):380–384
Matano M, Date S, Shimokawa M, et al. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat Med, 2015,21(3):256–262
Heckl D, Kowalczyk MS, Yudovich D, et al. Generation of mouse models of myeloid malignancy with combinatorial genetic lesions using CRISPR-Cas9 genome editing. Nat Biotechnol, 2014,32(9):941–946
Wang H, Yang H, Shivalila CS, et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell, 2013,153(4):910–918
Naldini L. Lentiviruses as gene transfer agents for delivery to non-dividing cells. Curr Opin Biotechnol, 1998,9(5):457–463
Stewar SA, Dykxhoorn DM, Palliser D, et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA, 2003,9(4):493–501
Kirnbauer R, Taub J, Greenstone H, et al. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J Virol, 1993,67(12):6929–6936
Zhou J, Sun XY, Stenzel DJ, et al. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology, 1991,185(1):251–257
Combelas N, Saussereau E, Fleury MJ, et al. Papillomavirus pseudovirions packaged with the L2 gene induce cross-neutralizing antibodies. J Transl Med, 2010,8:28
Fahey LM, Raff AB, Da Silva DM, et al. A major role for the minor capsid protein of human papillomavirus type 16 in immune escape. J Immunol, 2009,183(10):6151–6156
Buck CB, Pastrana DV, Lowy DR, et al. Efficient intracellular assembly of papillomaviral vectors. J Virol, 2004,78(2):751–757
Hung CF, Chiang AJ, Tsai HH, et al. Ovarian cancer gene therapy using HPV-16 pseudovirion carrying the HSV-tk gene. PLoS One, 2012,7(7):e40983–e40990
Kimchi-Sarfaty C, Vieira WD, Dodds D, et al. SV40 Pseudovirion gene delivery of a toxin to treat human adenocarcinomas in mice. Cancer Gene Ther, 2006,13(7):648–657
Author information
Authors and Affiliations
Corresponding author
Additional information
This project was supported by the National Natural Science Foundation of China (No. 81302273), Health and family Planning Commission of Hubei Province, China (No. WJ2015MB084), Health Department of Hubei Province, China ( No. 2012Z-Y02), and Science and Technology Department Support Project of Hubei Province, China (No. 2015BCA313).
Rights and permissions
About this article
Cite this article
Cheng, Yx., Chen, Gt., Yang, X. et al. Effects of HPV Pseudotype Virus in Cutting E6 Gene Selectively in SiHa Cells. CURR MED SCI 38, 212–221 (2018). https://doi.org/10.1007/s11596-018-1868-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-018-1868-3