Summary
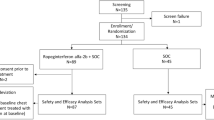
This study aims to explore the efficacy of interferon-α (IFN-α) combined with either entecavir (ETV) or adefovir (ADV) therapy versus IFN-α mono-therapy for chronic hepatitis B (CHB) patients, and to identify the factors associated with treatment outcomes. Totally, 159 CHB patients receiving interferon-based treatment for 48 weeks were enrolled in this retrospective study, including IFN-α mono-therapy group (group A, n=44), IFN-α plus ADV group (group B, n=53) and IFN-α plus ETV group (group C, n=62). The primary measures of efficacy assessments were the changes in HBsAg. Cox regression analysis was used to identify the predictors of treatment outcomes. The predictive values of the factors were assessed by ROC analysis. For patients with baseline hepatitis B surface antigen (HBsAg) level <1000 IU/mL, the reductions in mean HBsAg levels at week 48 were greater in group C than that in group A (P<0.05). Higher rate of HBeAg seroconversion was achieved in the combined therapy group than in IFN-α mono-therapy group at week 48 (P<0.05). Two factors were independently associated with HBeAg seroconversion: baseline HBeAg level <2.215 log10 index/mL and ΔHBeAg (decline in HBeAg from baseline) >0.175 log10 at week 12. In conclusion, interferon-α plus ETV therapy can accelerate HBsAg decline as compared with interferon-α mono-therapy in CHB patients with lower baseline HBsAg levels, and the combination therapy was superior to IFN-α mono-therapy in increasing the rate of HBeAg seroconversion. Baseline HBeAg and ΔHBeAg at week 12 can independently predict HBeAg seroconversion in patients subject to interferon-based therapy for 48 weeks.
Similar content being viewed by others
References
Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection. WHO Guidelines Approved by the Guidelines Review Committee. 2015, Geneva: World Health Organization
Wang FS, Fan JG, Zhang Z, et al. The global burden of liver disease: the major impact of China. Hepatology, 2014,60(6):2099–2108
Liaw YF, Kao JH, Piratvisuth T, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int, 2012,6(3):531–561
EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol, 2012,57(1):167–185
Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology, 2009,50(3):661–662
Zoulim F, Locarnini S. Hepatitis B virus resistance to nucleos(t)ide analogues. Gastroenterology, 2009,137(5):1593–1608
Reijnders JG, Perquin MJ, Zhang N, et al. Nucleos(t)ide analogues only induce temporary hepatitis B e antigen seroconversion in most patients with chronic hepatitis B. Gastroenterology, 2010,139(2):491–498
van Zonneveld M, Honkoop P, Hansen BE, et al. Long-term follow-up of alpha-interferon treatment of patients with chronic hepatitis B. Hepatology, 2004,39(3):804–810
Buster EH, Flink HJ, Cakaloglu Y, et al. Sustained HBeAg and HBsAg loss after long-term follow-up of HBeAg-positive patients treated with peginterferon alpha-2b. Gastroenterology, 2008,135(2):459–467
Fung J, Lai CL, Tanaka Y, et al. The duration of lamivudine therapy for chronic hepatitis B: cessation vs. continuation of treatment after HBeAg seroconversion. Am J Gastroenterol, 2009,104(8):1940–1946
Seto WK, Hui AJ, Wong VW, et al. Treatment cessation of entecavir in Asian patients with hepatitis B e antigen negative chronic hepatitis B: a multicentre prospective study. Gut, 2015,64(4):667–672
Marcellin P, Lau GK, Bonino F, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med, 2004,351(12):1206–1217
Janssen HL, van Zonneveld M, Senturk H, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet, 2005,365(9454):123–129
Piccolo P, Lenci I, Demelia L, et al. A randomized controlled trial of pegylated interferon-alpha2a plus adefovir dipivoxil for hepatitis B e antigen-negative chronic hepatitis B. Antivir Ther, 2009,14(8):1165–1174
Wursthorn K, Lutgehetmann M, Dandri M, et al. Peginterferon alpha-2b plus adefovir induce strong cccDNA decline and HBsAg reduction in patients with chronic hepatitis B. Hepatology, 2006,44(3):675–684
Ning Q, Han M, Sun Y, et al. Switching from entecavir to PegIFN alfa-2a in patients with HBeAg-positive chronic hepatitis B: a randomised open-label trial (OSST trial). J Hepatol, 2014,61(4):777–784
Brouwer WP, Xie Q, Sonneveld MJ, et al. Adding pegylated interferon to entecavir for hepatitis B e antigen-positive chronic hepatitis B: A multicenter randomized trial (ARES study). Hepatology, 2015,61(5):1512–1522
Zoutendijk R, Hansen BE, van Vuuren AJ, et al. Serum HBsAg decline during long-term potent nucleos(t)ide analogue therapy for chronic hepatitis B and prediction of HBsAg loss. J Infect Dis, 2011,204(3):415–418
Chevaliez S, Hezode C, Bahrami S, et al. Long-term hepatitis B surface antigen (HBsAg) kinetics during nucleoside/ nucleotide analogue therapy: finite treatment duration unlikely. J Hepatol, 2013,58(4):676–683
Zhao P, Liu W, Zhao J, et al. Comparison of the 48-week efficacy between entecavir and adefovir in HBeAg-positive nucleos(t)ide-naive Asian patients with chronic hepatitis B: a meta-analysis. Virol J, 2011,8:75
Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med, 2006,145(4):247–254
Hagiwara S, Kudo M, Osaki Y, et al. Impact of peginterferon alpha-2b and entecavir hydrate combination therapy on persistent viral suppression in patients with chronic hepatitis B. J Med Virol, 2013,85(6):987–995
Su WW, Hsu CW, Lee CM, et al. Combination therapy with peginterferon alfa-2a and a nucleos(t)ide analogue for HBeAg-positive chronic hepatitis B patients: results of a large, randomised, multicentre, double-blind, placebo-controlled study. J Hepatol, 2014,60(1 Suppl.):S47
HE Y, Tang XP, Zheng XH, et al. Therapeutic efficacy of combination therapy with interferon and a nucleoside analogue for treating chronic hepatitis B patients. J Clin Hepatol (Chinese), 2013,29(2):114–116
Lampertico P. The royal wedding in chronic hepatitis B: The haves and the have-nots for the combination of pegylated interferon and nucleos(t)ide therapy. Hepatology, 2015,61(5):1459–1461
Tangkijvanich P, Chittmittraprap S, Poovorawan K, et al. A randomized clinical trial of peginterferon alpha-2b with or without entecavir in patients with HBeAg-negative chronic hepatitis B: Role of host and viral factors associated with treatment response. J Viral Hepat, 2016,23(6):427–438
Huang R, Hao Y, Zhang J, et al. Interferon-alpha plus adefovir combination therapy versus interferon-alpha monotherapy for chronic hepatitis B treatment: A meta-analysis. Hepatol Res, 2013,43(10):1040–1051
Xie QL, Zhu Y, Wu LH, et al. The efficacy and safety of entecavir and interferon combination therapy for chronic hepatitis B virus infection: A meta-analysis. PLoS One, 2015,10(7):e132219
Boni C, Laccabue D, Lampertico P, et al. Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology, 2012,143(4):963–973
Tan AT, Hoang LT, Chin D, et al. Reduction of HBV replication prolongs the early immunological response to IFNalpha therapy. J Hepatol, 2014,60(1):54–61
Leung N, Peng CY, Hann HW, et al. Early hepatitis B virus DNA reduction in hepatitis B e antigen-positive patients with chronic hepatitis B: A randomized international study of entecavir versus adefovir. Hepatology, 2009,49(1):72–79
Chan HL, Wong VW, Chim AM, et al. Serum HBsAg quantification to predict response to peginterferon therapy of e antigen positive chronic hepatitis B. Aliment Pharmacol Ther, 2010,32(11-12): 1323–1331
Tseng TC, Liu CJ, Yang HC, et al. Determinants of spontaneous surface antigen loss in hepatitis B e antigen-negative patients with a low viral load. Hepatology, 2012,55(1):68–76
Sonneveld MJ, Rijckborst V, Boucher CA, et al. Prediction of sustained response to peginterferon alfa-2b for hepatitis B e antigen-positive chronic hepatitis B using on-treatment hepatitis B surface antigen decline. Hepatology, 2010,52(4):1251–1257
Takkenberg RB, Jansen L, de Niet A, et al. Baseline hepatitis B surface antigen (HBsAg) as predictor of sustained HBsAg loss in chronic hepatitis B patients treated with pegylated interferon-alpha2a and adefovir. Antivir Ther, 2013,18(7):895–904
Thompson AJ, Nguyen T, Iser D, et al. Serum hepatitis B surface antigen and hepatitis B e antigen titers: disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology, 2010,51(6):1933–1944
Xie Q, Zhou H, Bai X, et al. A randomized, open-label clinical study of combined pegylated interferon Alfa-2a (40KD) and entecavir treatment for hepatitis B “e” antigen-positive chronic hepatitis B. Clin Infect Dis, 2014,59(12):1714–1723
Marcellin P, Ahn SH, Ma X, et al. Combination of tenofovir disoproxil fumarate and peginterferon alpha-2a increases loss of hepatitis B surface antigen in patients with chronic hepatitis B. Gastroenterology, 2016,150(1):134–144
Author information
Authors and Affiliations
Corresponding authors
Additional information
This project was supported by grants from National Science and Technology Major Project for Infectious Diseases of China (No. 2013ZX10002001-001-006), the National Natural Science Foundation of China (No. 81461130019) and Deutsche Forschungsgemeinschaft (No. Transregio TRR60).
Rights and permissions
About this article
Cite this article
Li, H., Wang, H., Peng, C. et al. Predictors for efficacy of combination therapy with a nucleos(t)ide analogue and interferon for chronic hepatitis B. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 37, 547–555 (2017). https://doi.org/10.1007/s11596-017-1771-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-017-1771-3