Summary
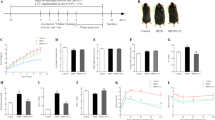
Dietary restriction (DR) can delay senescence, prolong lifespan of mammals and improve their learning-memory activity. The purpose of the study was to explore the effects of DR on hypolipidemic action and liver function of mice with hyperlipidemia. To investigate these effects, hyperlipidemia mouse models were established with high-fat diet (HFD) (34% of energy), then randomly divided into HFD group, DR30% group and DR50% group. Mice in DR30% and DR50% group were respectively supplied with HFD as much as about 70% and 50% of the consumption of HFD in the mice of HFD group. Rats in control group were fed routinely. After DR for 5 weeks, the average body weight, liver weight, liver index, serum lipids and glucose levels in both DR groups decreased significantly as compared with the HFD group (P<0.05 or P<0.01), so did alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH) levels and the ratio of LDL-C/HDL-C in the DR50% group (P<0.05 or P<0.01). Histopathology examination of liver tissues further proved ameliorative effect of DR on liver function. Western blotting showed that DR significantly increased the expression of silent mating type information regulation 2 homolog 1 (SIRT1) in liver and adipose, while notably decreased the expression of peroxisome proliferators-activated receptors-gamma (PPARγ) in adipose (P<0.05 or P<0.01). The increase of SIRT1 and decrease of PPARγ may be a mechanism by which DR reduces blood lipids and ameliorates liver function.
Similar content being viewed by others
References
Moore B. Symposium 1: overnutrition. consequences and solutions-non-alcoholic fatty liver disease: the hepatic consequences of obesity and the metabolic syndrome. Proc Nutr Soc, 2010,69: 211–220
Baek JH, Kim SJ, Kang HG, et al. Galectin-3 activates PPAR gamma and supports white adipose tissue formation and high-fat diet-induced obesity. Endocrinology, 2015, 156(1): 147–156
Kuo YH, Lin CH, Shih CC. Ergostatrien-3 beta-ol from antrodia camphorata inhibits diabetes and hyperlipidemia infat-diet treated mice via regulation of hepatic related genes, glucose transporter 4, and AMP-activated protein kinase phosphorylation. J Agr Food Chem, 2015,63(9): 2479–2489
Samartin S, Chandra RK. Obesity, overnutrition and the immune system. Nutr Res, 2001,21(1): 243–262
Lin S, Thomas T, Storlien L, et al. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int J Obesity, 2000,24(5): 639–646
Kohsaka A, Laposky AD, Ramsey KM, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab, 2007,6(5): 414–421
Hong Y, Deng C, Zhang J, et al. Neuroprotective effect of granulocyte colony-stimulating factor in a focal cerebral ischemic rat model with hyperlipidemia. J Huazhong Univ Sci Technol Med Sci, 2012,32(6): 872–878
Hipkiss AR. On the mechanisms of ageing suppression by dietary restriction-is persistent glycolysis the problem? Mech Ageing Dev, 2006,127(1): 8–15
Mair W, Goymer P, Pletcher SD, et al. Demography of dietary restriction and death in drosophila. Science, 2003,301(5640): 1731–1733
Lin SJ, Kaeberlein M, Andalis AA, et al. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature, 2002,418(6895): 344–348
Gao HT, Shao LX, Cheng WZ, et al. The effects of dietary restriction on weight, lipid-lowering and learning and memory ability in hyperlipidemia mice. Zhejiang Prev Med (Chinese), 2013,25(3): 1–4
Lin BQ, Zeng ZY, Yang SS, et al. Dietary restriction suppresses tumor growth, reduces angiogenesis, and improves tumor microenvironment in human non-small-cell lung cancer xenografts. Lung Cancer, 2013,79(2): 111–117
Gao HT, Shao LX, Sun GJ. Research advances in learning-memory and anti-tumor ability of dietary restriction. J Zhejiang Norm Univ (Nat Sci) (Chinese), 2014,37(2): 99–107
Devassy JG, Caligiuri SPB, Mayengbam S, et al. Dietary restriction in moderately obese rats improves body size and glucose handling without the renal and hepatic alterations observed with a high-protein diet. Appl Physiol Nutr Metab, 2015,40(4): 334–342
Fok WC, Bokov A, Gelfond J, et al. Combined treatment of rapamycin and dietary restriction has a larger effect on the transcriptome and metabolome of liver. Aging Cell, 2014,13(2): 311–319
Yu Z, Wang R, Fok WC, et al. Rapamycin and dietary restriction induce metabolically distinctive changes in mouse liver. J Gerontol A-Biol, 2015,70(4): 410–420
Jeon TI, Lim BO, Yu BP, et al. Effect of dietary restriction on age-related increase of liver susceptibility to peroxidation in rats. Lipids, 2001,36(6): 589–593
Tanaka K, Higami Y, Tsuchiya T, et al. Aging increases DNase gamma, an apoptosis-related endonuclease, in rat liver nuclei: effect of dietary restriction. Exp Gerontol, 2004,39(2): 195–202
Grattagliano I, Portincasa P, Cocco T, et al. Effect of dietary restriction and N-acetylcysteine supplementation on intestinal mucosa and liver mitochondrial redox status and function in aged rats. Exp Gerontol, 2004,39(9): 1323–1332
Gao HT, Shao LX, Cheng WZ, et al. Effects of dietary restriction on antioxidant capacity and Sirt1 expression in hyperlipideima mice. Acta Nutrimenta Sinica (Chinese), 2014,36(2): 168–173
Hamelet J, Demuth K, Paul JL, et al. Hyperhomo-cy-steinemia due to cystathionine beta synthase deficiency induces dysregulation of genes involved in hepatic lipid homeostasis in mice. J Hepatol, 2007,46(1): 151–159
Raval J, Lyman S, Nitta T, et al. Basal reactive oxygen species determine the susceptibility to apoptosis in cirrhotic hepatocytes. Free Radic Biol Med, 2006,41(11): 1645–1654
Wen H, Yang HJ, An YJ, et al. Enhanced phase II detoxification contributes to beneficial effects of dietary restriction as revealed by multi-platform metabolomics studies. Mol Cell Proteomics, 2013,12(3): 575–586
Aydin C, Ince E, Koparan S, et al. Protective effects of long term dietary restriction on swimming exercise-induced oxidative stress in the liver, heart and kidney of rat. Cell Biochem Funct, 2007,25(2): 129–137
Gao HT, Shao LX, Cheng WZ, et al. Effects of dietary restriction on lowing blood lipid and learning-memory behavious of hyperlipidemia mice. J Zhejiang Norm Univ (Nat Sci) (Chinese), 2013,36(3): 337–341
Someya S, Yu W, Hallows WC, et al. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell, 2010,143(5): 802–812
GB14924.3-2010 Laboratory animals-nutrients for formula feeds. Beijing: Standards Press of China, 2010
Huang YH, Zhang QH. Genistein reduced the neural apoptosis in the brain of ovariectomised rats by modulating mitochondrial oxidative stress. Brit J Nutr, 2010,104(09): 1297–1303
Li XR, Wang LJ, Li YH, et al. Polysorbates as novel lipid-modulating candidates for reducing serum total cholesterol and low-density lipoprotein levels in hyperlipidemic C57BL/6J mice and rats. Eur J Pharmacol, 2011,660(2-3):468–475
Beltaifa L, Chaouachi A, Zerifi R, et al. Walk-run transition speed training as an efficient exercise adjunct to dietary restriction in the management of obesity: a prospective intervention pilot study. Obes Facts, 2011,4(1): 45–52
Chen SD, Zhou HH, Lin MT, et al. Research of influence and mechanism of combining exercise with diet control on a model of lipid metabolism rat induced by high fat diet. Lipids Health Dis, 2013,12(1): 21–24
Gong WH, Zheng WX, Wang J, et al. Coexistence of hyperlipidemia and acute cerebral ischemia/reperfusion induces severe liver damage in a rat model. World J Gastroenterol, 2012,18(35): 4934–4943
Gao X, Wu JX, Dong ZY, et al. A low-protein diet supplemented with ketoacids plays a more protective role against oxidative stress of rat kidney tissue with 5/6 nephrectomy than a low-protein diet alone. Br J Nutr, 2010,103(4): 608–616
Grattagliano I, Palmieri VO, Portincasa P, et al. Oxidative stress-induced risk factors associated with the metabolic syndrome: a unifying hypothesis. J Nutr Biochem, 2008,19(8): 491–504
Yang XR, Wat E, Wang YP, et al. Effect of dietary cocoa tea (camellia ptilophylla) supplementation on high-fat diet-induced obesity, hepatic steatosis, and hyperlipidemia in mice. Evid Based Complement Alternat Med, 2013;2013:783860
Wu YZ, Yu YH, Szabo A, et al. Central inflammation and leptin resistance are attenuated by ginsenoside Rb1 treatment in obese mice fed a high-fat diet, PloS One, 2014,9(3):e92618
Hinnouho GM, Czernichow S, Dugravot A, et al. Metabolically healthy obesity and the risk of cardiovascular disease and type 2 diabetes: the Whitehall II cohort study. Eur Heart J, 2015,36(9): 551–559
Rodgers JT, Lerin C, Haas W, et al. Nutrient control of glucose homeostasis through a complex of PGC-1a and SIRT1. Nature, 2005,434(7029): 113–118
Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1a. Cell, 2006,127(6): 1109–1122
Pfluger PT, Herranz D, Velasco-Miguel S, et al. Sirt1 protects against high-fat diet-induced metabolic damage. P Natl Acad Sci USA, 2008,105(28): 9793–9798
Kitada M, Takeda A, Nagai T, et al. Dietary restriction ameliorates diabetic nephropathy through anti-inflamm-atory effects and regulation of the autophagy via restoration of Sirt1 in diabetic Wistar fatty (fa/fa) rats: a model of type 2 diabetes. Exp Diabetes Res, 2011, 2011: 908185
Inoue M, Ohtake T, Motomura W, et al. Increased expression of PPAR? in high fat diet-induced liver steatosis in mice. Biochem Bioph Res Co, 2005,336(1): 215–222
Jones JR, Barrick C, Kim K-A, et al. Deletion of PPAR? in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. P Natl Acad Sci USA, 2005,102(17): 6207–6212
Gu M, Fan S, Liu G, et al. Extract of wax gourd peel prevents high-fat diet-induced hyperlipidemia in C57BL/6 mice via the inhibition of the PPARgamma pathway. Evid Based Complement Alternat Med, 2013;2013:342561
Picard F, Kurtev M, Chung N, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-g-am-m-a. Nature, 2004,429(6993): 771–776
Linden MA, Lopez KT, Fletcher JA, et al. Combining metformin therapy with caloric restriction for the management of type 2 diabetes and nonalcoholic fatty liver disease in obese rats. Appl Physiol Nutr Metab, 2015,40(10): 1038–1047
Author information
Authors and Affiliations
Corresponding authors
Additional information
This project was supported by a grant from the Social Development Research Program of Science and Technology Agency of Jiangsu Province (No. BE2015646).
Rights and permissions
About this article
Cite this article
Gao, Ht., Cheng, Wz., Xu, Q. et al. Dietary restriction reduces blood lipids and ameliorates liver function of mice with hyperlipidemia. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 37, 79–86 (2017). https://doi.org/10.1007/s11596-017-1698-8
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-017-1698-8