Summary
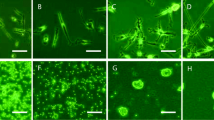
This study aimed to modify the mixed and purified culture of rat retinal ganglion cells (RGCs) in vitro. The retinae of 1–3 day old Sprague-Dawley (SD) rats were separated bluntly into two layers: inner layer and outer layer, under a surgical microscope. Retinal cells isolated from different layers (inner layer, outer layer and whole retinal tissue) by using enzyme dissociation method were cultured in F12/DMEM medium containing 15% FBS. After 3-day culture, the RGCs in the retinal cells obtained from mixed culture of inner, outer, and whole retinal tissue were identified by immunocytochemical staining of Thy-1.1, and the rate of RGCs to retinal cells (RGCs%) was calculated. Two monoclonal antibodies, anti-macrophages/granulocytes (OX-41) against rat macrophage and antibody against rat Thy-1.1 (OX-7), were used to purify RGCs by either a conventional or modified two-stepped immunopanning procedure (purification in situ). Purified RGCs were seeded at different cell density and cultured in F12/DMEM medium containing 15% FBS. Immunocytochemical staining for Thy-1.1, MTT, and PI-Hoechst33342 fluorescence imaging were used to identify the purity and the viability of RGCs in purified culture of RGCs. The results showed: (1) Immunocytochemistry of different retinal tissue layers culture revealed that the RGCs% was (19.9±1.2)%, (0.5±0.2)%, and (6.2±1.7)% respectively in the mixed culture of inner, outer, and whole retinal tissue, with differences being significant (P<0.05); (2) fluorescent double staining of Hoechst33342 and PI indicated that with the same RGCs%, RGCs obtained from purification in situ grew well with more neurite outgrowth than those by the conventional two-stepped immunopanning method; (3) the viability of purified RGCs seeded at high density was increased and the cells developed complex intercellular networks. The viability of RGCs was declined with the decreasing seeding density, and most cells presented round or oval in shape with thin neurites. It was concluded that: (1) RGCs% in the inner layer retina was higher than that in the outer layer retina; (2) RGCs obtained by in situ purification had more neurite outgrowth and lower mortality than those by conventional two-stepped immunopanning procedure; (3) the viability of purified RGCs could be increased by increasing cell seeding density to some extent.
Similar content being viewed by others
References
Farkas RH, Grosskreutz CL. Apoptosis, neuroprotection, and retinal ganglion cell death: an overview. Int Ophthalmol Clin, 2001,41(1):111–130
Levin LA. Mechanisms of optic neuropathy. Curr Opin Ophthalmol, 1997,8(6):9–15
Takahashi N, Cummins D, Caprioli J. Rat retinal ganglion cells in culture. Exp Eye Res, 1991,53(5):565–572
Lindsey JD, Weinreb RN. Survival and differentiation of purified retinal ganglion cells in a chemically defined microenvironment. Invest Ophthalmol Vis Sci, 1994, 35(10):3640–3648
Zhulin H, Shuhua D. Purification culture of retinal ganglion cells on two planes in assimilative culture solution in vitro. Chin J Ophthalmol (Chinese), 2002,38(7):440–441
Kashii S, Takahashi M, Mandai M, et al. Protective action of dopamine against glutamate neurotoxicity in the retina. Invest Ophthalmol Vis Sci, 1994,35(2):685–695
Barnstable CJ, Drager UC. Thy-1 antigen: A ganglion cells specific marker in rodent retina. Neuroscience, 1984, 11(4):847–855
Beale R, Osborne NN. Localization of the Thy-1 antigen to the surfaces of rat retinal ganglion cell. Neurochem Int, 1982,4(6):587–595
Barres BA, Silverstein BE, Corey DP, et al. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron, 1988, 1(9):791–803
Otori Y, Wei JY, Barnstable CJ. Neurotoxic effects of low doses of glutamate on purified rat retinal ganglion cells. Invest Ophthalmol Vis Sci, 1998,39(6):972–981
Shimazawa M, Yamashima T, Agarwal N. Neuroprotective effects of minocycline against in vitro and in vivo retinal ganglion cell damage. Brain Res, 2005,1053(1–2):185–194
Yisheng Z, Youqin J, Xiaoling X. Rat retinal ganglion cells in culture. Chin J Ophthalmol (Chinese), 1999,35(2): 137–139
Jin Y, Xiaoxin L. In vitro culture of retinal neuron cells of rat. Chin Ophthal Res (Chinese), 2004,22(4):340–342
Shiosaka S, Kiyama H, Tohyama M. A simple method for the separation of retinal sublayers from the entire retina with special reference to application for cell culture. J Neurosci Methods, 1984,10(3):229–235
Young RW. Cell differentiation in the retina of the mouse. Anat Rec, 1985,212(2):199–205
Marquardt T, Gruss P. Generating neuronal diversity in the retina: one for nearly all. Trends Neurosci, 2002,25(1): 32–38
Luo X, Lambrou GN, Sahel JA, et al. Hypoglycemia induces general neuronal death, whereas hypoxia and glutamate transport blockade lead to selective retinal ganglion cell death in vitro. Invest Ophthalmol Vis Sci, 2001, 42(11):2695–2705
Lipton SA, Leifer D, Barnstable CJ. Selectivity of Thy-1 monoclonal antibodies in enhancing neurite outgrowth. Neurosci Lett, 1992,137(1):75–77
Author information
Authors and Affiliations
Corresponding author
Additional information
This project was supported by a grant from National Natural Sciences Foundation of China (No. 30700922).
Rights and permissions
About this article
Cite this article
Xu, Z., Jiang, F., Zeng, Y. et al. Culture of rat retinal ganglion cells. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 31, 400–403 (2011). https://doi.org/10.1007/s11596-011-0389-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-011-0389-0