Summary
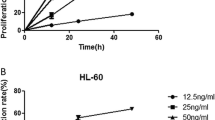
The inhibitory effect of wortmannin on leukemic cells and the possible mechanisms were examined. K562 cells were treated with wortmannin of various concentrations (3.125–100 nmol/L) for 0–72 h. MTT assay was used to evaluate the inhibitory effect of wortmannin on the growth of K562 cells. Cell apoptosis was detected by both Annexin-V FITC/PI double-labeled cytometry and transmission electron microscopy (TEM). The expression of p-Akt, T-p-Akt, NF-κBp65 and IKK-κB was determined by Western blotting and reverse transcription-polymerase chain reaction (RT-PCR). Our results showed that wortmannin obviously inhibited growth and induced apoptosis of K562 cells in vitro in a time- and dose-dependent manner. The IC50 value of wortmannin for 24 h was 25±0.14 nmol/L. Moreover, wortmannin induced K562 cells apoptosis in a dose-dependent manner. TEM revealed typical morphological changes of apoptosis in wortmannin-treated K562 cells, such as chromatin condensation, karyopyknosis, karyorhexis and apoptotic bodies. Additionally, several important intracellular protein kinases such as p-Akt, NF-κBp65 and IKK-κB experienced degradation of various degrees in a dose-dependent manner both at protein level and transcription level when cultured with wortmannin, but the expression of total Akt showed no change. It is concluded that wortmannin can inhibit the proliferation and induce apoptosis of K562 leukemia cells possibly by down-regulating the survival signaling pathways (PI3K/Akt and NF-κB channels).
Similar content being viewed by others
References
Olsen BB, Bjørling-Poulsen M, Guerra B. Emodin negatively affects the phosphoinositide 3-kinase/AKT signalling pathway: a study on its mechanism of action. Int J Biochem Cell Biol, 2007,39(1):227–237
Rudelius M, Pittaluga S, Nishizuka S, et al. Constitutive activation of Akt contributes to the pathogenesis and survival of mantle cell lymphoma. Blood, 2006,108(5): 1668–1676
Fillmore GC, Wang Q, Carey MJ, et al. Expression of Akt (protein kinase B) and its isoforms in malignant lymphomas. Leuk Lymphoma, 2005,46(12):1765–1773
Priulla M, Calastretti A, Bruno P, et al. Preferential chemosensitization of PTEN-mutated prostate cells by silencing the Akt kinase. Prostate, 2007,67(7):782–789
Martelli AM, Nyåkern M, Tabellini G, et al. Phosphoinositide 3-kinase/Akt signaling pathway and its theraeutical implications for human acute myeloid leukemia. Leukemia, 2006,20(6):91–98
Tang JM, He QY, Guo RX, et al. Phosphorylated Akt overexpression and loss of PTEN expression in non-small cell lung cancer confers poor prognosis. Lung Cancer, 2006,51(2):181–191
George P, Bali P, Annavarapu S, et al. Combination of the histone deacetylase inhibitor LBH589 and the hsp90 inhibitor 17-AAG is highly active against human CML-BC cells and AML cells with activating mutation of FLT-3. Blood, 2005,105(4):1768–1776
Liao Y, Grobholz R, Abel U, et al. Increase of Akt/PKB expression correlates with gleason pattern in human prostate cancer. Int J Cancer, 2003,107(4):676–680
Hsu J, Shi Y, Krajewski S, et al. The Akt kinase is activated in multiple myeloma tumor cells. Blood, 2001,98(9):2853–2855
Nakanishi K, Sakamoto M, Yamasaki S, et al. Akt phosphorylation is a risk factor for early disease recurrence and poor prognosis in hepatocellular carcinoma. Cancer, 2005, 103(2):307–312
Wipf P, Halter RJ. Chemistry and biology of wortmannin. Org Biomol Chem, 2005,3(11):2053–2061
Wu C, Huang J. Phosphatidylinositol 3-kinase/AKT mammalian target of rapamycin pathway is essential for neuroendocrine diferentiation of prostate cancer. J Biol Chem, 2007,282(6):3571–3583
Huang XL, Cui GH, Zhou KY, et al. Correlation of PI3K-Akt signal pathway to apoptosis of tumor cells. Chin J Cancer (Chinese), 2008,27(3):331–336
Grandage VL, Gale RE, Linch DC, et al. PI3-kinase/Akt is constitutively active in primary acute myeloid leukemia cells and regulates survival and chemoresistance via NF-κB, MAP kinase and p53 pathways. Leukemia, 2005, 19(4):586–594
Zhang C, Yang N, Zhang XW, et al. Advances in kinase inhibitors targeting PI3K/Akt-mTOR signal transduction pathway. Chin Oncol (Chinese), 2006,16(12):1064–1070
Xu Q, Simpson SE, Scialla TJ, et al. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood, 2003,102(3):972–980
Westfll SD, Skinner MK. Inhibition of phosphatidylinositol 3-kinase sensitizes ovarian cancer cells to carboplatin and allows adjunct chemotherapy treatment. Mol Cancer Ther, 2005,4(11):1764–1771
Panwalkar A, Verstovsek S, Giles FJ. Mammalian target of rapamyein inhibition as therapy for hematologic malignancies. Cancer, 2004,100(4):657–666
Xie X, Gao Q, Wang YL, et al. Inhibition of PI3 K/PKB signal pathway improves chemotherapeutic effect on gastric carcinoma cell lines. Chin Pharmacol Bull (Chinese), 2008,24(12):1666–1670
Karin M, Cao Y, Greten F R, et al. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer, 2002,2(4):301–310
Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest, 2001,107(3):241–246
Kaltschmidt B, Kaltschmidt C, Hofmann TG, et al. The pro- or anti-apoptotic function of NF-kappaB is determined by the nature of the apoptotic stimulus. Eur J Biochem, 2000,267(12):3828–3835
Bharti AC, Aggarwal BB. Nuclear factor-kappa B and cancer: its role in prevention and therapy. Biochem Pharmacol, 2002,64(5–6):883–888
Poh TW, Pervaiz S. LY294002 and LY303511 sensitize tumor cells to drug-induced apoptosis via intracellular hydrogen peroxide production independent of the phosphoinositide 3-kinase-Akt pathway. Cancer Res, 2005,65(14): 6264–6274
Ohta T, Ohnfiehi M, Hayasaka T, et al. Inhibition of phosphatidylinositol 3-kinase increases efficacy of cisplatin in vivo ovarian cancer models. Endocrinology, 2006,147(4): 1761–1769
Author information
Authors and Affiliations
Additional information
This project was supported by a grant from the National Natural Sciences Foundation of China (No. 30671743).
Rights and permissions
About this article
Cite this article
Wu, Q., Chen, Y., Cui, G. et al. Wortmannin inhibits K562 lukemic cells by regulating PI3k/Akt channel in vitro . J. Huazhong Univ. Sci. Technol. [Med. Sci.] 29, 451–456 (2009). https://doi.org/10.1007/s11596-009-0412-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-009-0412-x