Summary
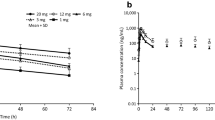
The current study was designed to determine the safety, tolerability and pharmacokinetic parameters of recombinant human parathyroid hormone [rhPTH (1–84)] used for the treatment of osteoporosis. In the single-dose format pharmacokinetic study, thirty-six healthy male volunteers received three dose levels of rhPTH (1–84) subcutaneously: 1, 2, and 4 μg/kg. The blood was timing drawn and the serum concentration of rhPTH (1–84) was determined by enzyme linked immunosorbent assay (ELISA). Serum concentration-time curves of PTH (1–84) exhibited a double-peak pattern, the first peak appearing about 10 to 30 min after administration and the second peak occurring about 1.5 to2 h after administration. Serum terminal half-time of PTH (1–84) was approximately 2 h. The parameters indicated the serum levels were directly proportional to the administered dose, with the mean Cmax and AUC0–24 ranging from approximately 543.47 to 1845 pg/mL and 2358.6 to 9232.12 pg·h·mL−1 over the dose range. The drug was well tolerated, the clinical symptoms were generally mild and of short duration.
Similar content being viewed by others
References
Habener JF, Rosenblatt M, Potts Jr, et al. Parathyroid hormone: biochemical aspects of biosynthesis, secretion, action, and metabolism. Physiol Rev, 1984,64(3): 985–1053
Liang CG, Li KJ, Zhang H, et al. Quality research of the recombinant human parathyroid hormone. Chin J Pharm Anal, 2005,25(2):215–219
Crandall C. Parathyroid hormone for treatment of osteoporosis. Arch Intern Med, 2002,162(20):2297–2309
Mannstadt M, Juppner H, Gradella TJ. Receptors for PTH and PTHrP: their biological importance and functional properties. Am J Physiol, 1999,277(5 Pt 2):F665–F675
MA DD, Ji GF, Xu ZH, et al. Studies on the secondary structure and function of PTH (1–34). Tianjin Yike Daxue Xuebao (Chinese), 2002,8(3):314–373
Gardella TJ, Jüppner H. Interaction of PTH and PTHrP with their receptors. Rev Endocr Metab Disord, 2000,1(4): 317–329
Warden SJ, Komatsu DE, Rydberg J, et al. Recombinant human parathyroid hormone (PTH 1–34) and low-intensity pulsed ultrasound have contrasting additive effects during fracture healing. Bone, 2009,44(3):485–494
Nakajima K, Nohtomi K, Sato M, et al. PTH (7–84) inhibits PTH (1–34)-induced 1, 25-(OH) 2D3 production in murine renal tubules. Biochem Biophys Res Commun, 2009,381(2):283–287
Tomida K, Hamano T, Mikami S, et al. Serum 25-hydroxyvitamin D as an independent determinant of 1–84 PTH and bone mineral density in non-diabetic predialysis CKD patients. Bone, 2009,44(4):678–683
Ueda K, Yamanaka Y, Harada, D et al. PTH has the potential to rescue disturbed bone growth in achondroplasia. Bone, 2007,41(1):13–18
Ogawa T, Yamagiwa H, Hayami T, et al. Human PTH(1–34) induces longitudinal bone growth in rats. J Bone Miner Metab 2002,20(2):83–90
LU GL, Zhang KQ, Tang W, et al. Effects of Parathyroid Hormone on the Expression of Transforming Growth Factor-B1 in Rats. Acta Univ Med Nanjing [Natural Science], 2003,24(4):371–372
Jin WF, Gu SZ, Gao JJ, et al. Effects of bone formation on culture osteoblasts with rhPTH (1–34) in vitro. Fudan Univ J Med Sci, 2006,33(6):815–818
Zeng WS, Liao HM, Yang ZP, et al. Analyse the amino acid composition of recombinant human parathyroid hormone 1–34. Pharm Biotechnol (Chinese), 2003,10(2): 108–111
Adis International Ltd. ALX111: ALX1-11, parathyroid hormone (1–84)-NPS Allelix, PREOS, PTH, recombinant human parathyroid hormone, rhPTH (1–84). Drug R D, 2003,4(4):231–235
Schwietert HR, Groen EWJ, Sollie FAE, et al. Single-dose subcutaneous administration of recombinant human parathyroid hormone [rhPTH (1–84)] in healthy postmenopausal volunteers. Clin Pharmacol Ther, 1997, 61:360–376
Hu Z, Niu H, Yang X, et al. Recombinant human parathyroid hormone 1–34: Pharmacokinetics, tissue distribution and excretion in rats. Inter J Pharm, 2006, 317(2):144–154
Groen EWJ, Schwietert HR, Van Marle SP. Multiple dose adminstration of recombinant human parathyroid hormone in healthy postmenopausal volunteers. Therapie, 1995,50(Suppl):525 (Abstract)
Lindsay R, Hodsman A, Genant H, et al. A randomized controlled multi-center study of 1–84 hPTH for treatment of postmenopausal osteoporosis. Bone, 1998,23(Suppl): 175
Rittmaster RS, Bolognese M, Ettinger MP, et al. Enhancement of bone mass in osteoporotic women with parathyroid hormone followed by alendronate. J Clin Endocr Metab, 2000,85(6):2129–2134
LU GC, She JH, Yuan BJ, et al. Pharmacological study on the safety of recombinant parathyroid hormone. Chin J New Drugs, 2003,12(10):833–836
Friedman PA, Goodman WG. PTH (1–84)/PTH (7–84): a balance of power. Am J Physiol Renal Physiol, 2006, 290(5):F975–F984
Kazama JJ, Omori T, Ei I, et al. Circulating 1–84 PTH and large C-terminal PTH fragment levels in uremia. Clin Exp Nephrol, 2003,7(2):114–119
Seibel MW, Lade DA, Hartke JR, et al. Validation and application of an immunoradiometric assay for the determination of human parathyroid hormone fragment 1–34 in dog plasma following subcutaneous and intravenous administration. J Pharm Biomed Anal, 1996, 14(12):1699–1707
Lindsay R, Zhou H, Cosman F, et al. Effects of a one-month treatment with PTH(1–34) on bone formation on cancellous, endocortical, and periosteal surfaces of the human ilium. J Bone Miner Res, 2007,22(4):495–502
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Li, Q., Qiao, J., Deng, J. et al. Safety, tolerability and pharmacokinetic study of recombinant human parathyroid hormone [rhPTH (1-84)] in Chinese healthy volunteers. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 29, 431–434 (2009). https://doi.org/10.1007/s11596-009-0408-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-009-0408-6