Summary
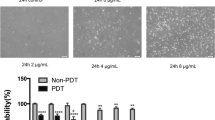
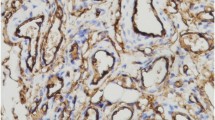
The effect of transfection of antisense vascular endothelial growth factor (VEGF) gene on the growth of hemangioma was studied. A total of 49 cases of capillary hemangiomas of the skin were collected. Immunohistochemical method was used to detect the expression of PCNA in hemangioma tissues. According to the finding, 49 cases of hemangiomas fell into proliferating phase (27 cases) and involuting phase (22 cases) respectively. Another 5 cases of normal skin tissues adjacent to the tumor tissues served as control. Immunohistochemical staining was performed to detect the expression of VEGF in the tumor tissues and the normal tissues. The average absorbance (A) values and the average positive area rate of VEGF were measured by image analysis system (HPIAS-2000). Endothelial cells from the tumor tissues in proliferating phase were cultured. Eukaryotic expression vector was constructed by sub-cloning, and transfected into human hemangioma endothelial cells by using cation liposome as vector. The expression of VEGF mRNA and protein was detected by RT-PCR and indirect immunofluorescence assay (IFA), respectively, and the biological characteristics of the transfected endothelial cells were examined by MTT assay and flow cytometry (FCM) after transfection. Immunohistochemical results showed that the expression of VEGF in proliferating endothelial cells was remarkably higher than those in involuting endothelial cells and normal endothelial cells (P<0.01), but there was no significant difference in the expression of VEGF between involuting endothelial cells and normal ones (P>0.01). Electrophoresis and sequencing indicated that the eukaryotic expression vector containing antisense VEGF gene, i.e. pcDNA3.1-VEGF, was successfully constructed. After VEGF antisense RNA recombinant was transfected into hemangioma endothelial cells, RT-PCR revealed that the expression of VEGF mRNA in pcDNA-VEGF (V) group and blank group was obviously higher than that in pcDNA-VEGF (A) group, and that the expression of endogenous VEGF mRNA in pcDNA-VEGF (A) group was significantly inhibited. Immunohistochemical result demonstrated that, compared with blank group, there was statistically significant difference between pcDNA-VEGF (A) and pcDNA-VEGF (V) groups (P<0.01), but there was no significant difference between pcDNA-VEGF (V) group and blank group (P>0.05). The activity of endothelial cell proliferation was reduced significantly after transfection, and obvious apoptosis occurred in hemangioma endothelial cells after transfection of antisense VEGF. It was suggested that VEGF plays an important role in the pathological change of hemangiomas by promoting endothelial cell proliferation and angiogenesis. Antisense VEGF gene transfection could effectively inhibit the growth of hemanioma endothelial cells.
Similar content being viewed by others
References
Giatromanolaki A, Arvanitidou V, Hatzimichael A, et al. The HIF-2alpha/VEGF pathway activation in cutaneous capillary haemangiomas. Pathology, 2005, 37(2):149–151
Zhang L, Lin X, Wang W, et al. Circulating level of vascular endothelial growth factor in differentiating hemangioma from vascular malformation patients. Plast Reconstr Surg, 2005,116(1):200–204
Marchuk DA. Pathogenesis of hemangioma. J Clin Invest, 2001,107(6):665–666
Yu Y, Flint AF, Mulliken JB, et al. Endothelial progenitor cells in infantile hemangioma. Blood, 2004,103(4):1373–1375
Rini BI. VEGF-targeted therapy in metastatic renal cell carcinoma. Oncologist, 2005,10(3):191–197
Matsui T, Ono T, Kito M, et al. Extensive facial strawberry mark associated with cerebellar hypoplasia and vascular abnormalities. J dermatol, 1997,24(2):113–116
Yu Y, Flint AF, Mulliken JB, et al. Endothelial progenitor cells in infantile hemangioma. Blood, 2004,103(4): 1373–1375
Petrova TV, Makinen T, Alitalo K. Signaling via vascular endothelial growth factor receptors. Exp Cell Res, 1999, 253(1):117–130
Matsuno A, Nagashima T. Specific gene suppression using antisense strategy for growth suppression of glioma. Med Electron Microsc, 2004,37(3):158–161
Gu ZP, Wang YJ, Wu Y, et al. Synthesis of ribozyme against vascular endothelial growth factor165 and its biological activity in vitro. World J Gastroenterol, 2004, 10(10):1495–1498
Glade-Bender J, Kandel JJ, Yamashiro DJ. VEGF blocking therapy in the treatment of cancer. Expert Opin Biol Ther, 2003,3(2):263–276
Underiner TL, Ruggeri B, Gingrich DE. Development of vascular endothelial growth factor receptor (VEGFR) kinase inhibitors as anti-angiogenic agents in cancer therapy. Curr Med Chem, 2004,11(6):731–745
Dvorak AM, Feng D. The vesiculo-vacuolar organelle (VVO). A new endothelial cell permeability organelle. Histochem Cytochem, 2001,49(4):419–432
Qu-Hong, Nagy JA, Senger DR. Ultrastructural localization of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) to the abluminal plasma membrane and vesiculovacuolar organelles of tumor microvascular endothelium. Histochem Cytochem, 1995, 43(4):381–389
Trisciuoglio D, Iervolino A, Zupi G, et al. Involvement of PI3K and MAPK signaling in bcl-2-induced vascular endothelial growth factor expression in melanoma cells. Mol Biol Cell, 2005,6(9):4153–4162
Kumar P, Miller AI, Polverini PJ. MAPK mediates gamma-irradiation-induced endothelial cell apoptosis, and vascular endothelial growth factor protects endothelial cells through the phosphoinositide 3-kinase-Akt-Bcl-2 pathway. J Biol Chem, 2004,279(41):43352–43360
Nor JE, Christensen J, Mooney DJ, et al. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol, 1999, 154(2):375–384
Author information
Authors and Affiliations
Additional information
This project was supported by grants from the National Natural Sciences Foundation of China (No. 30872688), Hubei Province Sci-tech Bureau Department (No. 2003ABA164, 2004ABA155 and 2004AA301c107), and Hubei Province Hygiene Department (No. JXIB075).
Rights and permissions
About this article
Cite this article
Shan, S., Shan, G. & Zhang, D. Treatment of hemangioma by transfection of antisense VEGF gene. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 29, 335–339 (2009). https://doi.org/10.1007/s11596-009-0314-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-009-0314-y